S-777,469
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
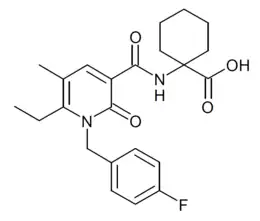
| Formula | C23H27FN2O4 |
| Molar mass | 414.477 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
S-777,469 is a drug developed by Shionogi which is a cannabinoid receptor agonist, with 128x selectivity for the CB2 subtype, having a CB2 affinity of 36nM, and a CB1 affinity over 4600nM. In animal studies it showed antipruritic effects, and passed Phase II human trials for the treatment of atopic dermatitis, but development was ultimately not continued further.[1][2][3][4]
See also
References
- ↑ Odan M, Ishizuka N, Hiramatsu Y, Inagaki M, Hashizume H, Fujii Y, et al. (April 2012). "Discovery of S-777469: an orally available CB2 agonist as an antipruritic agent". Bioorganic & Medicinal Chemistry Letters. 22 (8): 2803–6. doi:10.1016/j.bmcl.2012.02.072. PMID 22444677.
- ↑ Haruna T, Soga M, Morioka Y, Hikita I, Imura K, Furue Y, et al. (2015). "S-777469, a novel cannabinoid type 2 receptor agonist, suppresses itch-associated scratching behavior in rodents through inhibition of itch signal transmission". Pharmacology. 95 (1–2): 95–103. doi:10.1159/000371890. PMID 25721168. S2CID 41617054.
- ↑ Haruna T, Soga M, Morioka Y, Imura K, Furue Y, Yamamoto M, et al. (2017). "The Inhibitory Effect of S-777469, a Cannabinoid Type 2 Receptor Agonist, on Skin Inflammation in Mice". Pharmacology. 99 (5–6): 259–267. doi:10.1159/000455916. PMID 28214870. S2CID 31692497.
- ↑ Bow EW, Rimoldi JM (2016). "The Structure-Function Relationships of Classical Cannabinoids: CB1/CB2 Modulation". Perspectives in Medicinal Chemistry. 8: 17–39. doi:10.4137/PMC.S32171. PMC 4927043. PMID 27398024.
External links
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.