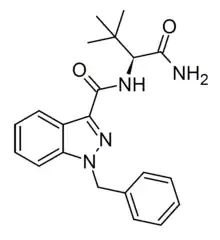
ADB-BINACA
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C21H24N4O2 |
| Molar mass | 364.449 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
ADB-BINACA is a cannabinoid designer drug that has been found as an ingredient in some synthetic cannabis products.[1] It was originally developed by Pfizer as a potential analgesic, and is a potent agonist of the CB1 receptor with a binding affinity (Ki) of 0.33 nM and an EC50 of 14.7 nM.[2]
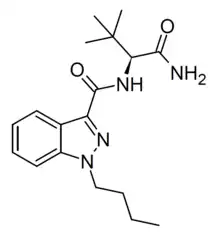
ADB-BUTINACA
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C18H26N4O2 |
| Molar mass | 330.432 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
The analogue with a 1-butyl substitution on the indazole ring rather than 1-benzyl has also been sold as a designer drug under the name ADB-BINACA, but is now more commonly referred to as ADB-BUTINACA to avoid confusion with the benzyl compound.[3][4][5] It is a similarly potent CB1 agonist, with an EC50 of 6.36 nM.[6][7]
See also
References
- ↑ Qian Z, Hua Z, Liu C, Jia W (2016). "Four types of cannabimimetic indazole and indole derivatives, ADB-BINACA, AB-FUBICA, ADB-FUBICA, and AB-BICA, identified as new psychoactive substances". Forensic Toxicology. 34: 133–143. doi:10.1007/s11419-015-0297-2. PMC 4705129. PMID 26793280.
- ↑ WO 2009106982, "Indazole Derivatives"
- ↑ Kavanagh P, Pechnikov A, Nikolaev I, Dowling G, Kolosova M, Grigoryev A (August 2021). "Detection of ADB-BUTINACA Metabolites in Human Urine, Blood, Kidney and Liver". Journal of Analytical Toxicology. doi:10.1093/jat/bkab088. PMID 34341821.
- ↑ Sia CH, Wang Z, Goh EM, Tan YL, Fong CY, Moy HY, Chan EC (November 2021). "Urinary Metabolite Biomarkers for the Detection of Synthetic Cannabinoid ADB-BUTINACA Abuse". Clinical Chemistry. 67 (11): 1534–1544. doi:10.1093/clinchem/hvab134. PMID 34387654.
- ↑ Kronstrand R, Norman C, Vikingsson S, Biemans A, Valencia Crespo B, Edwards D, et al. (November 2021). "The metabolism of the synthetic cannabinoids ADB-BUTINACA and ADB-4en-PINACA and their detection in forensic toxicology casework and infused papers seized in prisons". Drug Testing and Analysis. doi:10.1002/dta.3203. PMID 34811926. S2CID 244490343.
- ↑ Cannaert A, Sparkes E, Pike E, Luo JL, Fang A, Kevin RC, et al. (December 2020). "Synthesis and in Vitro Cannabinoid Receptor 1 Activity of Recently Detected Synthetic Cannabinoids 4F-MDMB-BICA, 5F-MPP-PICA, MMB-4en-PICA, CUMYL-CBMICA, ADB-BINACA, APP-BINACA, 4F-MDMB-BINACA, MDMB-4en-PINACA, A-CHMINACA, 5F-AB-P7AICA, 5F-MDMB-P7AICA, and 5F-AP7AICA". ACS Chemical Neuroscience. 11 (24): 4434–4446. doi:10.1021/acschemneuro.0c00644. PMID 33253529. S2CID 227246346.
- ↑ Pike E, Grafinger KE, Cannaert A, Ametovski A, Luo JL, Sparkes E, et al. (July 2021). "Systematic evaluation of a panel of 30 synthetic cannabinoid receptor agonists structurally related to MMB-4en-PICA, MDMB-4en-PINACA, ADB-4en-PINACA, and MMB-4CN-BUTINACA using a combination of binding and different CB1 receptor activation assays: Part I-Synthesis, analytical characterization, and binding affinity for human CB1 receptors". Drug Testing and Analysis. 13 (7): 1383–1401. doi:10.1002/dta.3037. PMID 33787091.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.