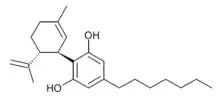
Cannabidiphorol
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C23H34O2 |
| Molar mass | 342.523 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
The heptyl homologue of cannabidiol was identified as a natural phytocannabinoid and named cannabidiphorol (CBDP) in 2019.[1] It had previously been reported as a synthetic compound,[2] but was not identified as a natural product prior to 2019.
References
- ↑ Citti C, Linciano P, Russo F, Luongo L, Iannotta M, Maione S, et al. (December 2019). "A novel phytocannabinoid isolated from Cannabis sativa L. with an in vivo cannabimimetic activity higher than Δ9-tetrahydrocannabinol: Δ9-Tetrahydrocannabiphorol". Scientific Reports. 9 (1): 20335. doi:10.1038/s41598-019-56785-1. PMC 6937300. PMID 31889124.
- ↑ Makriyannis A, et al. Angiogenic resorcinol derivatives. US Patent application 2012/172339
See also
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.