JWH-138
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C24H36O2 |
| Molar mass | 356.550 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
JWH-138 (THC-Octyl, Δ8-THC-C8) is a synthetic cannabinoid first synthesised by John W. Huffman, with a Ki of 8.5nM at the CB1 cannabinoid receptor.[1]
Isomers
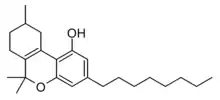
Δ3-THC-C8
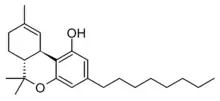
Δ9-THC-C8, CAS# 2552798-63-5
The Δ3/Δ6a(10a) isomer was synthesised in 1941, but was found to be slightly less active than Δ3-THC itself.[2] The alternate isomer Δ9-THC-C8 has also been synthesised,[3] and both the Δ8 and Δ9 isomers are included within the definition of an "intoxicating cannabinoid" in Colorado under the name tetrahydrocannabioctyl,[4] but it is unclear if it has been identified as a natural product. Tetrahydrocannabioctyl is sometimes referred to as THC-Octyl or THCO, which may cause confusion with THC-O-acetate which is commonly known as THC-O.
See also
References
- ↑ Martin BR, Jefferson R, Winckler R, Wiley JL, Huffman JW, Crocker PJ, et al. (September 1999). "Manipulation of the tetrahydrocannabinol side chain delineates agonists, partial agonists, and antagonists". The Journal of Pharmacology and Experimental Therapeutics. 290 (3): 1065–1079. PMID 10454479.
- ↑ Adams R, Loewe S, Jelinek C, Wolff H (July 1941). "Tetrahydrocannabinol Homologs with Marihuana Activity. IX". Journal of the American Chemical Society. 63 (7): 1971–1973. doi:10.1021/ja01852a052.
- ↑ WO application 2020232545, Abdur-Rashid, Kamaluddin; Jia, Wenli & Abdur-Rashid, Kareem, "Catalytic cannabinoid processes and precursors", published 2020-11-26, assigned to Kare Chemical Technologies Inc..
- ↑ Senate Bill 23-271, General Assembly, State of Colorado
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.