GAT100
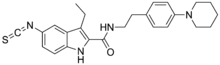 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C25H28N4OS |
| Molar mass | 432.59 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
GAT100 is a negative allosteric modulator of the cannabinoid CB1 receptor.[1][2]
See also
References
- ↑ Kulkarni PM, Kulkarni AR, Korde A, Tichkule RB, Laprairie RB, Denovan-Wright EM, et al. (January 2016). "Novel Electrophilic and Photoaffinity Covalent Probes for Mapping the Cannabinoid 1 Receptor Allosteric Site(s)". Journal of Medicinal Chemistry. 59 (1): 44–60. doi:10.1021/acs.jmedchem.5b01303. PMC 4716578. PMID 26529344.
- ↑ Laprairie RB, Kulkarni AR, Kulkarni PM, Hurst DP, Lynch D, Reggio PH, et al. (June 2016). "Mapping Cannabinoid 1 Receptor Allosteric Site(s): Critical Molecular Determinant and Signaling Profile of GAT100, a Novel, Potent, and Irreversibly Binding Probe". ACS Chemical Neuroscience. 7 (6): 776–98. doi:10.1021/acschemneuro.6b00041. PMC 5358098. PMID 27046127.
| Phytocannabinoids |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endocannabinoids |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthetic cannabinoid receptor agonists / neocannabinoids |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Allosteric CBR ligands |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Endocannabinoid enhancers (inactivation inhibitors) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Anticannabinoids (antagonists/inverse agonists/antibodies) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Receptor (ligands) |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (modulators) |
| ||||||||||||
| Enzyme (modulators) |
| ||||||||||||
| Others |
| ||||||||||||
| |||||||||||||
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.