BMS-F
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| Chemical and physical data | |
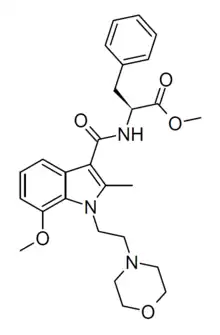
| Formula | C27H33N3O5 |
| Molar mass | 479.577 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
BMS-F is a chemical from the aminoalkylindole family invented by Bristol-Myers Squibb around 1999,[1] that acts as a potent and selective agonist for the cannabinoid receptor CB2, with a Ki of 8 nM at CB2 and 500x selectivity over the related CB1 receptor. It has antiinflammatory effects and inhibits release of TNF-α.[2][3]
See also
- A-796,260
- APP-FUBINACA
- JWH-200
- MDMB-FUBINACA
- MN-25
- Pravadoline
- S-777,469
- WIN 55,212-2
References
- ↑ WO application 0158869, Leftheris K, Zhao R, Chen BC, Kiener P, Wu H, Pandit CR, Wrobleski S, Chen P, Hynes J, Longphre M, Norris DJ, Spergel S, Tokarski J, "Cannabinoid Receptor Modulators, Their Processes of Preparation, and use of Cannabinoid Receptor Modulators for Treating Respiratory and Non-Respiratory Diseases", published 16 August 2001, assigned to Bristol-Myers Squibb
- ↑ Hynes Jr J, Leftheris K, Wu H, Pandit C, Chen P, Norris DJ, et al. (September 2002). "C-3 Amido-indole cannabinoid receptor modulators". Bioorganic & Medicinal Chemistry Letters. 12 (17): 2399–2402. doi:10.1016/s0960-894x(02)00466-3. PMID 12161142.
- ↑ Howlett AC, Thomas BF, Huffman JW (October 2021). "The Spicy Story of Cannabimimetic Indoles". Molecules. 26 (20). doi:10.3390/molecules26206190. PMID 34684770.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.