Cannabigerolic acid
 | |
| Names | |
|---|---|
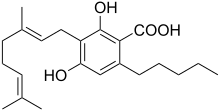
| Preferred IUPAC name
3-[(2E)-3,7-Dimethylocta-2,6-dien-1-yl]-2,4-dihydroxy-6-pentylbenzoic acid | |
| Identifiers | |
CAS Number |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
| Properties | |
Chemical formula |
C22H32O4 |
| Molar mass | 360.494 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cannabigerolic acid (CBGA) is the acidic form of cannabigerol (CBG). It is a dihydroxybenzoic acid and olivetolic acid in which the hydrogen at position 3 is substituted by a geranyl group. It is a biosynthetic precursor to Delta-9-tetrahydrocannabinol, which is the principal psychoactive constituent of the Cannabis plant. It is also a diterpenoid, a polyketide, a member of resorcinols and a phytocannabinoid. It derives from an olivetolic acid. It is a conjugate acid of a cannabigerolate.[1]
In the Cannabis plant, olivetolic acid and geranyl diphosphate are synthesized into CBGA.[2][3] CBGA is converted in the plant by CBCA synthase, CBDA synthase and THCA synthase into CBCA, CBDA and THCA respectively.[4] Afterwards, THCA and CBDA can be decarboxylated into THC and CBD by drying and heating plant material.
References
- ↑ PubChem 2020.
- ↑ Thomas & ElSohly 2015, p. 6.
- ↑ Degenhardt, Stehle & Kayser 2016, p. 17.
- ↑ Thomas & ElSohly 2015, pp. 6–7.
Sources
- "Compound Summary – cannabigerolic acid". PubChem. United States National Library of Medicine. Retrieved April 7, 2020.
- Thomas, Brian F.; ElSohly, Mahmoud A. (2015). The Analytical Chemistry of Cannabis: Quality Assessment, Assurance, and Regulation of Medicinal Marijuana and Cannabinoid Preparations. Emerging Issues in Analytical Chemistry. Elsevier Science. ISBN 978-0-12-804670-8.
- Degenhardt, F.; Stehle, F.; Kayser, O. (2016). "The biosynthesis of cannabinoids". In Preedy, Victor R. (ed.). Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis, and Treatment. Academic Press. pp. 13–23. ISBN 978-0128008270.
Further reading
- Havelka, Jacqueline (September 9, 2019). "What is CBGA (Cannabigerolic Acid) & what does this cannabinoid do?". Leafly.