PF-4840154
 | |
| Names | |
|---|---|
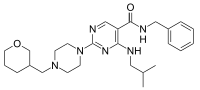
| Preferred IUPAC name
N-Benzyl-4-[(2-methylpropyl)amino]-2-{4-[(oxan-3-yl)methyl]piperazin-1-yl}pyrimidine-5-carboxamide | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C26H38N6O2 |
| Molar mass | 466.630 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
PF-4840154 is a pyrimidine derivative discovered by Pfizer at its Sandwich, Kent research center. The compound is a potent, selective activator of both the human (EC50 = 23 nM) and rat (EC50 = 97 nM) TRPA1 channels.[1] This compound elicits nociception in a mouse model through TRPA1 activation. PF-4840154 is used as a reference agonist of the TRPA1 channel for in-vitro high-throughput screening purposes, and is superior to allyl isothiocyanate for this use.[2] The TRPA1 channel is considered an attractive pain target based on the fact that TRPA1 knockout mice showed near complete attenuation of pain behaviors in some pre-clinical development models.[3][4]
See also
References
- ↑ Samanta, Amrita; Kiselar, Janna; Pumroy, Ruth A.; Han, Seungil; Moiseenkova-Bell, Vera Y. (2018-04-27). "Structural insights into the molecular mechanism of mouse TRPA1 activation and inhibition". Journal of General Physiology. Rockefeller University Press. 150 (5): 751–762. doi:10.1085/jgp.201711876. ISSN 0022-1295. PMC 5940248. PMID 29703838.
- ↑ Ryckmans T, Aubdool AA, Bodkin JV, Cox P, Brain SD, Dupont T, Fairman E, Hashizume Y, Ishii N, Kato T, Kitching L, Newman J, Omoto K, Rawson D, Strover J (July 2011). "Design and pharmacological evaluation of PF-4840154, a non-electrophilic reference agonist of the TrpA1 channel". Bioorg. Med. Chem. Lett. 21 (16): 4857–4859. doi:10.1016/j.bmcl.2011.06.035. PMID 21741838.
- ↑ McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM (August 2007). "TRPA1 mediates formalin-induced pain". Proc. Natl. Acad. Sci. U.S.A. 104 (33): 13525–30. doi:10.1073/pnas.0705924104. PMC 1941642. PMID 17686976.
- ↑ McMahon SB, Wood JN (March 2006). "Increasingly irritable and close to tears: TRPA1 in inflammatory pain". Cell. 124 (6): 1123–5. doi:10.1016/j.cell.2006.03.006. PMID 16564004.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.