SB-366791
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
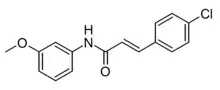
| Formula | C16H14ClNO2 |
| Molar mass | 287.74 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
SB-366791 is a drug which acts as a potent and selective blocker of the TRPV1 ion channel. It has analgesic effects in animal studies, and is used in research into pain and inflammation.[1][2][3]
See also
References
- ↑ Dell H (December 2003). "New compound fires up pain research". Drug Discovery Today. 8 (23): 1053. doi:10.1016/s1359-6446(03)02928-3. PMID 14693457.
- ↑ Ma SX, Kim HC, Lee SY, Jang CG (December 2018). "TRPV1 modulates morphine self-administration via activation of the CaMKII-CREB pathway in the nucleus accumbens". Neurochemistry International. 121: 1–7. doi:10.1016/j.neuint.2018.10.009. PMID 30292787. S2CID 52929149.
- ↑ Uchytilova E, Spicarova D, Palecek J (April 2021). "Hypersensitivity Induced by Intrathecal Bradykinin Administration Is Enhanced by N-oleoyldopamine (OLDA) and Prevented by TRPV1 Antagonist". International Journal of Molecular Sciences. 22 (7): 3712. doi:10.3390/ijms22073712. PMC 8038144. PMID 33918267.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.