Tetrahydrocannabiphorol
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C23H34O2 |
| Molar mass | 342.523 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
(-)-Trans-Δ9-tetrahydrocannabiphorol (Δ9-THCP, (C7)-Δ9-THC, and THC-Heptyl), is a potent phytocannabinoid, a CB1 and CB2 agonist which was known as a synthetic homologue of THC,[1] but for the first time in 2019 was isolated as a natural product in trace amounts from Cannabis sativa. It is structurally similar to Δ9-THC, the main active component of cannabis, but with the pentyl side chain extended to heptyl. Since it has a longer side chain, its cannabinoid effects are "far higher than Δ9-THC itself." It is said to have at least 30 times higher affinity to cannabinoid receptors than THC.[2] The binding activity of Delta-9-THCP against human CB1 receptor in vitro is Ki = 1.2 nM.[3] and the binding activity of Delta-8-THCP against human CB1 receptor in vitro is Ki = 22 nM.[4]
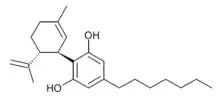
The corresponding heptyl homologue of cannabidiol was also identified in the same study, and named cannabidiphorol (CBDP).[3]
The Δ8 isomer is also known as a synthetic cannabinoid under the code name JWH-091 but should not be confused with other JWH compounds of unrelated structure classes such as JWH-018, an indole which structurally unrelated to JWH-091 but happens to include JWH in its nickname. ,[5][4] It's unconfirmed whether or not Delta-8-THCP is found naturally in cannabis plants, but likely is due to Delta-8-THC itself being a degraded form of Delta-9-THC. [6] JWH-091 has approximately double the binding affinity at the CB1 receptor (22nM ± 3.9nM) in comparison to Delta-9-THC (40.7nM ± 1.7nM) or Delta-8-THC (44nM ± 12nM).[4] but appears significantly lower in vitro than the binding activity of Delta-9-THCP (Ki = 1.2 nM CB1)[3]
See also
References
- ↑ Harvey DJ (March 1985). "Identification of hepatic metabolites of n-heptyl-delta-1-tetrahydrocannabinol in the mouse". Xenobiotica; the Fate of Foreign Compounds in Biological Systems. 15 (3): 187–97. doi:10.3109/00498258509045349. PMID 2992174.
- ↑ "THCp and CBDp, Newly Discovered, Extremely Potent Cannabinoids". 29 June 2020.
- 1 2 3 Citti C, Linciano P, Russo F, Luongo L, Iannotta M, Maione S, et al. (December 2019). "A novel phytocannabinoid isolated from Cannabis sativa L. with an in vivo cannabimimetic activity higher than Δ9-tetrahydrocannabinol: Δ9-Tetrahydrocannabiphorol". Scientific Reports. 9 (1): 20335. doi:10.1038/s41598-019-56785-1. PMC 6937300. PMID 31889124.
- 1 2 3 Bow EW, Rimoldi JM (2016). "The Structure-Function Relationships of Classical Cannabinoids: CB1/CB2 Modulation". Perspectives in Medicinal Chemistry. 8: 17–39. doi:10.4137/PMC.S32171. PMC 4927043. PMID 27398024.
- ↑ Martin BR, Jefferson R, Winckler R, Wiley JL, Huffman JW, Crocker PJ, et al. (September 1999). "Manipulation of the tetrahydrocannabinol side chain delineates agonists, partial agonists, and antagonists". The Journal of Pharmacology and Experimental Therapeutics. 290 (3): 1065–79. PMID 10454479.
- ↑ Hazekamp, Arno; Fischedick, Justin T.; Díez, Mónica Llano; Lubbe, Andrea; Ruhaak, Renee L. (2010). "Chemistry of Cannabis". Comprehensive Natural Products II. pp. 1033–1084. doi:10.1016/B978-008045382-8.00091-5. ISBN 9780080453828.
