Etrinabdione
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C28H35NO3 |
| Molar mass | 433.592 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
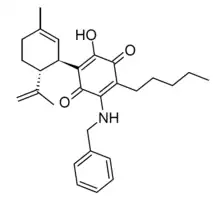
Etrinabdione (VCE-004.8, EHP-101) is a drug structurally related to cannabidiol and HU-331 which has potent antiinflammatory and neuroprotective effects. It acts as a dual agonist of CB2 and PPARγ and is in clinical trials for the treatment of scleroderma.[1][2][3][4][5][6][7][8][9]
See also
References
- ↑ Navarrete C, Carrillo-Salinas F, Palomares B, Mecha M, Jiménez-Jiménez C, Mestre L, et al. (March 2018). "Hypoxia mimetic activity of VCE-004.8, a cannabidiol quinone derivative: implications for multiple sclerosis therapy". Journal of Neuroinflammation. 15 (1): 64. doi:10.1186/s12974-018-1103-y. PMC 5831753. PMID 29495967.
- ↑ García-Martín A, Garrido-Rodríguez M, Navarrete C, Del Río C, Bellido ML, Appendino G, et al. (November 2018). "EHP-101, an oral formulation of the cannabidiol aminoquinone VCE-004.8, alleviates bleomycin-induced skin and lung fibrosis". Biochemical Pharmacology. 157: 304–313. doi:10.1016/j.bcp.2018.07.047. PMID 30076848. S2CID 51921018.
- ↑ Palomares B, Ruiz-Pino F, Navarrete C, Velasco I, Sánchez-Garrido MA, Jimenez-Jimenez C, et al. (October 2018). "VCE-004.8, A Multitarget Cannabinoquinone, Attenuates Adipogenesis and Prevents Diet-Induced Obesity". Scientific Reports. 8 (1): 16092. Bibcode:2018NatSR...816092P. doi:10.1038/s41598-018-34259-0. PMC 6208444. PMID 30382123.
- ↑ García-Martín A, Garrido-Rodríguez M, Navarrete C, Caprioglio D, Palomares B, DeMesa J, et al. (May 2019). "Cannabinoid derivatives acting as dual PPARγ/CB2 agonists as therapeutic agents for systemic sclerosis". Biochemical Pharmacology. 163: 321–334. doi:10.1016/j.bcp.2019.02.029. PMID 30825431. S2CID 73492817.
- ↑ Navarrete C, García-Martin A, Garrido-Rodríguez M, Mestre L, Feliú A, Guaza C, et al. (September 2020). "Effects of EHP-101 on inflammation and remyelination in murine models of Multiple sclerosis". Neurobiology of Disease. 143: 104994. doi:10.1016/j.nbd.2020.104994. hdl:10261/219645. PMID 32599064. S2CID 220075831.
- ↑ Burgaz S, García C, Gómez-Cañas M, Rolland A, Muñoz E, Fernández-Ruiz J (May 2021). "Neuroprotection with the Cannabidiol Quinone Derivative VCE-004.8 (EHP-101) against 6-Hydroxydopamine in Cell and Murine Models of Parkinson's Disease". Molecules. 26 (11): 3245. doi:10.3390/molecules26113245. PMC 8198479. PMID 34071302.
- ↑ Caprioglio D, Mattoteia D, Taglialatela-Scafati O, Muñoz E, Appendino G (July 2021). "Cannabinoquinones: Synthesis and Biological Profile". Biomolecules. 11 (7): 991. doi:10.3390/biom11070991. PMC 8301883. PMID 34356614.
- ↑ Navarrete C, García-Martín A, Correa-Sáez A, Prados ME, Fernández F, Pineda R, et al. (July 2022). "A cannabidiol aminoquinone derivative activates the PP2A/B55α/HIF pathway and shows protective effects in a murine model of traumatic brain injury". Journal of Neuroinflammation. 19 (1): 177. doi:10.1186/s12974-022-02540-9. PMC 9270745. PMID 35810304.
- ↑ Lavayen BP, Yang C, Larochelle J, Liu L, Tishko RJ, de Oliveira AC, et al. (May 2023). "Neuroprotection by the cannabidiol aminoquinone VCE-004.8 in experimental ischemic stroke in mice". Neurochemistry International. 165: 105508. doi:10.1016/j.neuint.2023.105508. PMID 36863495. S2CID 257235882.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.