CP 42,096
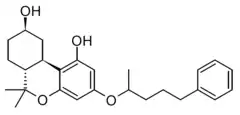 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C26H34O4 |
| Molar mass | 410.554 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
CP 42,096 is an analgesic drug which acts as a cannabinoid agonist. It was developed by Pfizer in the 1980s as part of the research that led to the development of levonantradol,[1][2][3] and is more potent than THC but less potent than newer compounds such as CP 55,244.[4][5]
See also
References
- ↑ Howlett AC, Johnson MR, Melvin LS, Milne GM (March 1988). "Nonclassical cannabinoid analgetics inhibit adenylate cyclase: development of a cannabinoid receptor model". Molecular Pharmacology. 33 (3): 297–302. PMID 3352594.
- ↑ Prescott WR, Martin BR (1990). "The evaluation of synthetic cannabimimetic congeners for discriminative stimulus and cataleptogenic effects in rats". NIDA Research Monograph. 105: 421. OCLC 7457082. PMID 1652087.
- ↑ Koe BK (1999). "Levonantradol". In Nahas GG, Sutin KM, Harvey D, Agurell S, Pace N, Cancro R (eds.). Marihuana and Medicine. Totowa, NJ: Humana Press. pp. 553–560. doi:10.1007/978-1-59259-710-9_53. ISBN 978-1-4757-5717-0.
- ↑ Koe BK, Milne GM, Weissman A, Johnson MR, Melvin LS (February 1985). "Enhancement of brain [3H]flunitrazepam binding and analgesic activity of synthetic cannabimimetics". European Journal of Pharmacology. 109 (2): 201–12. doi:10.1016/0014-2999(85)90421-2. PMID 2986995.
- ↑ "Hexahydrocannabinol (HHC) and related substances" (PDF). European Monitoring Centre for Drugs and Drug Addiction. 2023.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.