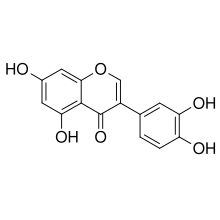
Orobol
 | |
 | |
| Names | |
|---|---|
| IUPAC name
3′,4′,5,7-Tetrahydroxyisoflavone | |
| Preferred IUPAC name
3-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4H-1-benzopyran-4-one | |
| Other names
Isoluteolin Santol 5,7,3',4'-Tetrahydroxyisoflavone | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
Beilstein Reference |
292790 |
| ChEBI | |
| ChemSpider | |
| MeSH | D011794 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C15H10O6 |
| Molar mass | 286.23 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Orobol is one of several known isoflavones. It can be isolated from Aspergillus niger or Streptomyces neyagawaensis. It is a potent inhibitor of Phosphoinositide 3-kinase.[1][2]
References
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.