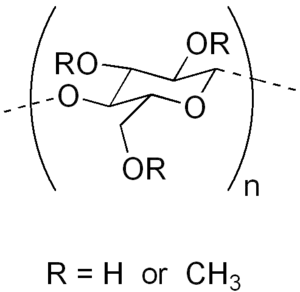
Methyl cellulose
 | |
| Names | |
|---|---|
| Other names | Cellulose, methyl ether; methylated cellulose; methylcellulose; E461 |
| Clinical data | |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Chemical and physical data | |
| Formula | variable |
| Molar mass | variable |
Methyl cellulose (or methylcellulose) is a chemical compound derived from cellulose. It is sold under a variety of trade names and is used as a thickener and emulsifier in various food and cosmetic products, and also as a bulk-forming laxative. Like cellulose, it is not digestible, not toxic, and not an allergen.
In 2017, it was the 272nd most commonly prescribed medication in the United States, with more than one million prescriptions.[1][2]
Uses
Methyl cellulose has a wide range of uses.
Medical
Constipation
Methyl cellulose is used to treat constipation.[3] Effects generally occur within three days.[3] It is taken by mouth and is recommended with sufficient water.[4] Side effects may include abdominal pain.[4] It is classified as a bulk forming laxative.[3] It works by increasing the amount of stool present which improves intestinal contractions.[4]
It is available over the counter.[3] It is sold under the brand name Citrucel among others.[3]
Artificial tears and saliva
The lubricating property of methyl cellulose is of particular benefit in the treatment of dry eyes.[5] Solutions containing methyl cellulose or similar cellulose derivatives are used as substitute for tears or saliva if the natural production of these fluids is disturbed.
Medication manufacturing
Methyl cellulose is used in the manufacture of drug capsules; its edible and nontoxic properties provide a vegetarian alternative to the use of gelatin.
Consumer products
Thickener and emulsifier
Methyl cellulose is very occasionally added to hair shampoos, tooth pastes and liquid soaps, to generate their characteristic thick consistency. This is also done for foods, for example ice cream or croquette. Methyl cellulose is also an important emulsifier, preventing the separation of two mixed liquids because it is an emulsion stabilizer.
Methyl cellulose is also used as paint rheological modifier to prevent paint sagging.
Food
The E number of methyl cellulose as food additive is E461. E464 is hydroxypropylcellulose and more soluble in water.[6]
Methyl cellulose, as a gel, has the unique property of setting when hot and melting when cold.[7]
Methyl cellulose is used as an ingredient in some meat analogues that are intended to replicate the texture of meat.[8]
Lubricant
Methyl cellulose may be used in personal lubricant.
Construction materials
Methyl cellulose finds a major application as a performance additive in construction materials. It is added to mortar dry mixes to improve the mortar's properties such as workability, open and adjustment time, water retention, viscosity, adhesion to surfaces etc. Construction grade methyl cellulose is not to be identified with food and pharmaceutical grade methyl cellulose and hydroxypropyl methyl cellulose, as it may be cross-linked with glyoxal for easy dispersion in water.
The construction materials can be cement-based or gypsum-based. Notable examples of dry mixture mortars which utilize methyl cellulose include tile adhesives, EIFS, insulating plasters, hand-troweled and machine-sprayed plaster, stucco, self-leveling flooring, extruded cement panels, skim coats, joint & crack fillers, and tile grouts. Typical usage is about 0.2% – 0.5% of total dry powder weight for dry mixtures.
Derivatives of methyl cellulose which improve performance characteristics include hydroxypropyl methyl cellulose (HPMC) and hydroxyethyl methyl cellulose (HEMC). These derivatives typically improve the characteristics such as water retention, vertical surface slip resistance, open time, etc.
Glue and binder
Methyl cellulose can be employed as a mild glue which can be washed away with water. This may be used in the fixing of delicate pieces of art as well as in book conservation to loosen and clean off old glue from spines and bookboards.
Methyl cellulose is the main ingredient in many wallpaper pastes. It is also used as a binder in pastel crayons and also as a binder in medications.
Paper and textile sizing
Methyl cellulose is used as sizing in the production of papers and textiles as it protects the fibers from absorbing water or oil.
Cell culture
Methyl cellulose is also used in cell culture to study viral replication. It is dissolved in the same nutrient-containing medium in which cells are normally grown. A single layer of cells is grown on a flat surface, then infected with a virus for a short time. The strength of the viral sample used will determine how many cells get infected during this time. The thick methyl cellulose medium is then added on top of the cells in place of normal liquid medium. As the viruses replicate in the infected cells, they are able to spread between cells whose membranes touch each other, but are trapped when they enter the methyl cellulose. Only cells closely neighboring an infected cell will become infected and die. This leaves small regions of dead cells called plaques in a larger background of living uninfected cells. The number of plaques formed is determined by the strength of the original sample.
Bacterial and protozoal motility inhibitor
Aqueous methyl cellulose solutions have been used to slow bacterial and protozoal cell motility for closer inspection. Changing the amount of methyl cellulose in solution permits the adjustment of the solution's viscosity.
Stem cell differentiation
Methyl cellulose is used in the most common approaches to quantify multiple or single lineage-committed hematopoietic progenitors, called colony-forming cells (CFCs) or colony-forming units (CFUs), in combination with culture supplements that promote their proliferation and differentiation, and allow the clonal progeny of a single progenitor cell to stay together and thus form a colony of more mature cells.
Chemistry
It is a hydrophilic white powder in pure form and dissolves in cold (but not in hot) water, forming a clear viscous solution or gel.
Methyl cellulose is used as a buffer additive in capillary electrophoresis to control electroosmotic flow for improved separations.
Special effects
The slimy, gooey appearance of an appropriate preparation of methyl cellulose with water, in addition to its nontoxic, nonallergenic, and edible properties, makes it popular for use in special effects for motion pictures and television wherever vile slimes must be simulated. In the film Ghostbusters, the gooey substance the supernatural entities used to "slime" the Ghostbusters was mostly a thick water solution of methyl cellulose. The Aliens ooze and drip a great deal of methyl cellulose—especially the queen.
Methyl cellulose has been used to safely simulate molten materials, as well. In several of the Terminator films, it was back-lit with colored gels and films to reproduce the heated glow of iron in the large pouring ladles used to transport the metal from the smelting ovens to the various molds and forms. Methyl cellulose was also a stand-in for the lava flows in Los Angeles in Volcano and on the volcanic surface of Mustafar, in Star Wars: Episode III – Revenge of the Sith.
Chemistry
Methyl cellulose does not occur naturally and is synthetically produced by heating cellulose with caustic solution (e.g. a solution of sodium hydroxide) and treating it with methyl chloride. In the substitution reaction that follows, the hydroxyl residues (-OH functional groups) are replaced by methoxide (-OCH3 groups).
Different kinds of methyl cellulose can be prepared depending on the number of hydroxyl groups substituted. Cellulose is a polymer consisting of numerous linked glucose molecules, each of which exposes three hydroxyl groups. The Degree of Substitution (DS) of a given form of methyl cellulose is defined as the average number of substituted hydroxyl groups per glucose. The theoretical maximum is thus a DS of 3.0, however more typical values are 1.3–2.6.
Different methyl cellulose preparations can also differ in the average length of their polymer backbones.
Solubility and temperature
Methyl cellulose has a lower critical solution temperature (LCST) between 40 °C and 50 °C. At temperatures below the LCST, it is readily soluble in water; above the LCST, it is not soluble, which has a paradoxical effect that heating a saturated solution of methyl cellulose will turn it solid, because methyl cellulose will precipitate out. The temperature at which this occurs depends on DS-value, with higher DS-values giving lower solubility and lower precipitation temperatures because the polar hydroxyl groups are masked.
Preparing a solution of methyl cellulose with cold water is difficult however: as the powder comes into contact with water, a gel layer forms around it, dramatically slowing the diffusion of water into the powder, hence the inside remains dry. A better way is to first mix the powder with hot water, so that the methyl cellulose particles are well dispersed (and so have a much higher effective surface area) in the water, and cool down this dispersion while stirring, leading to the much more rapid dissolution of those particles.
Society and culture
Cost
In 2017, it was the 272nd most commonly prescribed medication in the United States, with more than one million prescriptions.[1][2]
.svg.png.webp) Methyl cellulose prescriptions (US)
Methyl cellulose prescriptions (US).svg.png.webp) Methyl cellulose costs (US)
Methyl cellulose costs (US)
See also
- Carboxymethyl cellulose
- Ethyl cellulose
- Ethyl methyl cellulose
- Hydroxypropyl cellulose
- Hydroxyethyl cellulose
References
- 1 2 "The Top 300 of 2017". ClinCalc.
{{cite web}}:|access-date=requires|url=(help); Missing or empty|url=(help) - 1 2 "Methylcellulose (4000 Mpa.S) - Drug Usage Statistics". ClinCalc. Archived from the original on 8 July 2020. Retrieved 11 April 2020.
- 1 2 3 4 5 "DailyMed - methylcellulose powder, for solution". dailymed.nlm.nih.gov. Archived from the original on 28 August 2021. Retrieved 19 April 2019.
- 1 2 3 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 54. ISBN 9780857113382.
- ↑ Sandford-Smith, John (1995). Eye Diseases In Hot Climates. ELBS British Government.
- ↑ Younes, Maged; Aggett, Peter; Aguilar, Fernando; Crebelli, Riccardo; Domenico, Alessandro Di; Dusemund, Birgit; Filipič, Metka; Frutos, Maria Jose; Galtier, Pierre; Gott, David; Gundert‐Remy, Ursula; Kuhnle, Gunter Georg; Lambré, Claude; Leblanc, Jean-Charles; Lillegaard, Inger Therese; Moldeus, Peter; Mortensen, Alicja; Oskarsson, Agneta; Stankovic, Ivan; Tobback, Paul; Waalkens‐Berendsen, Ine; Wright, Matthew; Tard, Alexandra; Tasiopoulou, Stavroula; Woutersen, Rudolf Antonius (2018). "Re-evaluation of celluloses E 460(i), E 460(ii), E 461, E 462, E 463, E 464, E 465, E 466, E 468 and E 469 as food additives". EFSA Journal. 16 (1): e05047. doi:10.2903/j.efsa.2018.5047. PMC 7009359. PMID 32625652. S2CID 80411182.
- ↑ Blumenthal, Heston (19 November 2004). "The Appliance of Science (Melting Point)". The Guardian. Archived from the original on 17 September 2014. Retrieved 8 August 2012.
- ↑ Erica, Chayes Wida (4 February 2020). "Do Impossible and Beyond Meat burgers really contain laxatives?". TODAY.com. Archived from the original on 15 November 2020. Retrieved 2 September 2020.
Further reading
- Cathleen Baker (1984). Methylcellulose & Sodium Carboxymethylcellulose: An Evaluation for Use in Paper Conservation through Accelerated Aging. Preprints of the Contributions to the Paris Congress, 2–8 September 1984: Adhesives and Consolidants, pp. 55–59. International Institute of Conservation.
- Robert L. Feller and Myron H. Wilt (1990). Evaluation of Cellulose Ethers for Conservation (free fulltext). Getty Conservation Institute. Archived from the original on 2014-05-12. Retrieved 2014-05-10.
- Pijper A (March 1947). "Methylcellulose and Bacterial Motility". J. Bacteriol. 53 (3): 257–69. doi:10.1128/JB.53.3.257-269.1947. PMC 518306. PMID 16561270.
External links
| Identifiers: |
|---|