Carboxymethyl cellulose
 | |
 | |
| Names | |
|---|---|
| Other names
Carboxymethylcellulose; carmellose; E466 | |
| Identifiers | |
CAS Number |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider |
|
| UNII | |
| Properties | |
Chemical formula |
variable |
| Molar mass | variable |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
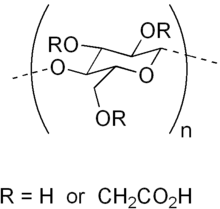
Carboxymethyl cellulose (CMC) or cellulose gum[1] is a cellulose derivative with carboxymethyl groups (-CH2-COOH) bound to some of the hydroxyl groups of the glucopyranose monomers that make up the cellulose backbone. It is often used as its sodium salt, sodium carboxymethyl cellulose. It used to be marketed under the name Tylose, a registered trademark of SE Tylose.
Preparation
It is synthesized by the alkali-catalyzed reaction of cellulose with chloroacetic acid.[2] The polar (organic acid) carboxyl groups render the cellulose soluble and chemically reactive.
Following the initial reaction, the resultant mixture produces about 60% CMC plus 40% salts (sodium chloride and sodium glycolate). This product is the so-called technical CMC which is used in detergents. A further purification process is used to remove these salts to produce the pure CMC used for food, pharmaceutical, and dentifrice (toothpaste) applications. An intermediate "semipurified" grade is also produced, typically used in paper applications such as restoration of archival documents.
The functional properties of CMC depend on the degree of substitution of the cellulose structure (i.e., how many of the hydroxyl groups have taken part in the substitution reaction), as well as the chain length of the cellulose backbone structure and the degree of clustering of the carboxymethyl substituents.
Uses
CMC is used in food under the E number E466 or E469 (when it is enzymatically hydrolyzed) as a viscosity modifier or thickener, and to stabilize emulsions in various products including ice cream. It is also a constituent of many non-food products, such as toothpaste, laxatives, diet pills, water-based paints, detergents, textile sizing, reusable heat packs, and various paper products. It is used primarily because it has high viscosity, is nontoxic, and is generally considered to be hypoallergenic as the major source fiber is either softwood pulp or cotton linter.[3][4] CMC is used extensively in gluten free and reduced fat food products.[5] In laundry detergents, it is used as a soil suspension polymer designed to deposit onto cotton and other cellulosic fabrics, creating a negatively charged barrier to soils in the wash solution. In opthalmology, CMC is used as a lubricant in artificial tears to treat dry eyes. Extensive treatment may be required to treat severe dry eye syndrome or Meibomian gland dysfunction (MGD).
CMC is also used as a thickening agent, for example, in the oil-drilling industry as an ingredient of drilling mud, where it acts as a viscosity modifier and water retention agent. Sodium CMC(Na CMC) for example, is used as a negative control agent for alopecia in rabbits.
Knitted fabric made of cellulose (e.g. cotton or viscose rayon) may be converted into CMC and used in various medical applications.
- Device for epistaxis (nose bleeding). A poly-vinyl chloride (PVC) balloon is covered by CMC knitted fabric reinforced by nylon. The device is soaked in water to form a gel, this is inserted into the nose and the balloon inflated. The combination of the inflated balloon and the therapeutic effect of the CMC stops the bleeding.
- Fabric used as a dressing following ear nose and throat surgical procedures.
- Water is added to form a gel, and this gel is inserted into the sinus cavity following surgery.
Insoluble microgranular CMC is used as a cation-exchange resin in ion-exchange chromatography for purification of proteins.[6] Presumably, the level of derivatization is much lower, so the solubility properties of microgranular cellulose are retained, while adding sufficient negatively charged carboxylate groups to bind to positively charged proteins.
CMC is also used in ice packs to form a eutectic mixture resulting in a lower freezing point, and therefore more cooling capacity than ice.[7]
Aqueous solutions of CMC have also been used to disperse carbon nanotubes. The long CMC molecules are thought to wrap around the nanotubes, allowing them to be dispersed in water. In conservation-restoration, it is used as an adhesive or fixative (commercial name Walocel, Klucel).
CMC is used to achieve tartrate or cold stability in wine. This innovation may save megawatts of electricity used to chill wine in warm climates. It is more stable than metatartaric acid and is very effective in inhibiting tartrate precipitation. It is reported that KHT crystals, in presence of CMC, grow slower and change their morphology.[8] Their shape becomes flatter because they lose 2 of the 7 faces, changing their dimensions. CMC molecules, negatively charged at wine pH, interact with the electropositive surface of the crystals, where potassium ions are accumulated. The slower growth of the crystals and the modification of their shape are caused by the competition between CMC molecules and bitartrate ions for binding to the KHT crystals (Cracherau et al. 2001).
In veterinary medicine, CMC is used in abdominal surgeries in large animals, particularly horses, to prevent the formation of bowel adhesions.
CMC is sometimes used as an electrode binder in advanced battery applications (i.e. lithium ion batteries), especially with graphite anodes. CMC's water solubility allows for less toxic and costly processing than with non-water-soluble binders, like the traditional polyvinylidene fluoride (PVDF), which requires toxic n-methylpyrrolidone (NMP) for processing. CMC is often used in conjunction with styrene-butadiene rubber (SBR) for electrodes requiring extra flexibility, e.g. for use with silicon-containing anodes.[9]
Culinary uses
CMC powder is widely used in the ice cream industry, to make ice creams without churning or extreme low temperatures, thereby eliminating the need for the conventional churners or salt ice mixes.[10] CMC is used in preparing bakery products such as bread and cake. The use of CMC gives the loaf a much improved quality at a reduced cost to the baker, by economizing on the fat component. CMC is also used as an emulsifier in high quality biscuits. By dispersing fat uniformly in the dough, it improves the release of the dough from the moulds and cutters, achieving well-shaped biscuits without any distorted edges. It can also help to reduce the amount of egg yolk or fat used in making the biscuits, thus achieving economy. Use of CMC in candy preparation ensures smooth dispersion in flavour oils, and improves texture and quality. CMC is used in chewing gums, margarines and peanut butter as an emulsifier. It is also used in leather crafting to burnish the edges.[11]
Enzymology
CMC has also been used extensively to characterize enzyme activity from endoglucanases (part of the cellulase complex). CMC is a highly specific substrate for endo-acting cellulases, as its structure has been engineered to decrystallize cellulose and create amorphous sites that are ideal for endoglucanase action. CMC is desirable because the catalysis product (glucose) is easily measured using a reducing sugar assay, such as 3,5-dinitrosalicylic acid. Using CMC in enzyme assays is especially important in regard to screening for cellulase enzymes that are needed for more efficient cellulosic ethanol conversion. However, CMC has also been misused in earlier work with cellulase enzymes, as many had associated whole cellulase activity with CMC hydrolysis. As the mechanism of cellulose depolymerization has become better understood, exo-cellulases are dominant in the degradation of crystalline (e.g. Avicel) and not soluble (e.g. CMC) cellulose.
Side effects
While thought to be uncommon, case reports of severe reactions to carboxymethyl cellulose exist. In one such instance, a woman was known to experience anaphylaxis following exposure.[12] Skin testing is believed to be a useful diagnostic tool for this purpose.[12]
Effects on inflammation, microbiota-related metabolic syndrome, and colitis are a subject of research.[13] Carboxymethyl cellulose has been found to cause inflammation of the gut, altering microbiota, and was found to be a triggering factor of inflammatory bowel diseases such as ulcerative colitis and Crohn's disease.[14]
Society and culture
Cost
Carboxymethyl cellulose sodium 0.5% is used for ocular purposes as artificial tears[15]The U.S. cost is about $16.49 (USD) for 0.5 fl. oz.[16][17]
.svg.png.webp) Carboxymethyl cellulose costs (US)
Carboxymethyl cellulose costs (US).svg.png.webp) Carboxymethyl cellulose prescriptions (US)
Carboxymethyl cellulose prescriptions (US)
See also
- Croscarmellose sodium
- Hydroxypropyl cellulose
- Methyl cellulose
References
- ↑ Codex Alimentarius Commission (2016). "Sodium carboxymethyl cellulose (Cellulose gum)". GFSA Online. FAO. Archived from the original on 2017-09-12. Retrieved 2017-05-08.
- ↑ Hollabaugh, C. B.; Burt, Leland H.; Walsh, Anna Peterson (October 1945). "Carboxymethylcellulose. Uses and Applications". Industrial & Engineering Chemistry. 37 (10): 943–947. doi:10.1021/ie50430a015.
- ↑ "CP Kelco Cellulose Gum / Carboxymethyl Cellulose". Archived from the original on 2013-08-24. Retrieved 2013-07-17.
- ↑ "Sodium Carboxymethylcellulose - The Ideal Hydrocolloid for Bakery & Dough Products" (PDF). Archived from the original (PDF) on 2015-06-26.
- ↑ Stanford, John (January 2012). "Food Processing Technologies for Reduction of Fat in Products" (PDF). Food & Health Innovation Service. Scotland Food & Drink. Archived from the original (PDF) on 2014-10-23.
- ↑ "Whatman Filters & Sample Collection". Archived from the original on 2 May 2013. Retrieved 9 November 2016.
- ↑ Use in ice packs Archived July 8, 2011, at the Wayback Machine
- ↑ Gerbaud, Vincent (18 October 1996). Determination de l'etat de sursaturation et effet des polysaccharides sur la cristallisation du bitartrate de potassium dans les vins [Determination of the state of supersaturation and effect of polysaccharides on the crystallization of potassium bitartrate in wines] (PDF) (Ph.D.) (in français). Institut National Polytechnique de Talouse. Docket 961NP1030G. Archived (PDF) from the original on 2016-10-13. Retrieved 2017-05-07.
- ↑ Archived 2017-12-04 at the Wayback Machine Applications of sodium carboxymethyl cellulose As a Binder In Batteries
- ↑ Bahramparvar, Maryam; Mazaheri Tehrani, Mostafa (October 2011). "Application and Functions of Stabilizers in Ice Cream". Food Reviews International. 27 (4): 389–407. doi:10.1080/87559129.2011.563399. S2CID 43187328.
- ↑ "C.m.c. Glossary - Recipes with C.m.c. - Tarladalal.com". Archived from the original on 15 December 2016. Retrieved 9 November 2016.
- 1 2 Lieberman, Phil. "Anaphylaxis to carboxymethylcellulose". American Academy of Allergy, Asthma, and Immunology. Archived from the original on 2017-07-12. Retrieved 2017-07-12.
- ↑ Healy, Melissa (2015-02-25). "Is common food additive to blame for rising rates of bowel disease?". Los Angeles Times. Archived from the original on 2017-07-12. Retrieved 2017-07-12.
- ↑ Martino, John Vincent; Van Limbergen, Johan; Cahill, Leah E. (1 May 2017). "The Role of Carrageenan and Carboxymethylcellulose in the Development of Intestinal Inflammation". Frontiers in Pediatrics. 5: 96. doi:10.3389/fped.2017.00096. PMC 5410598. PMID 28507982.
- ↑ "carboxymethylcellulose sodium ophthalmic (eye): Uses, Side Effects, Interactions, Pictures, Warnings & Dosing - WebMD". www.webmd.com. Archived from the original on 2 December 2020. Retrieved 25 March 2021.
- ↑ "OPTIVE LUBRICANT EYE DROPS - CVS Pharmacy". www.cvs.com. Archived from the original on 28 August 2021. Retrieved 26 March 2021.
- ↑ "DailyMed - CVS LUBRICANT EYE DROPS- carboxymethylcellulose sodium liquid". dailymed.nlm.nih.gov. Archived from the original on 28 August 2021. Retrieved 26 March 2021.
External links
- CMC chemical structure and properties
- MC and CMC: commercial preparations and various uses, including paper conservation; bibliography Archived 2020-06-22 at the Wayback Machine