Inflammatory bowel disease
| Inflammatory bowel diseases | |
|---|---|
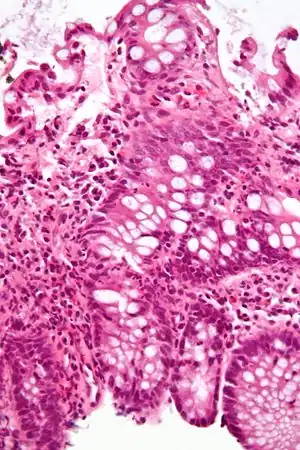 | |
| Micrograph showing inflammation of the large bowel in a case of inflammatory bowel disease. Colonic biopsy. H&E stain. | |
| Specialty | Gastroenterology |
| Differential diagnosis | Gastroenteritis, irritable bowel syndrome, celiac disease |
| Frequency | 11.2 million worldwide (2015)[1] |
| Deaths | 47,400 worldwide (2015)[2] |
Inflammatory bowel disease (IBD) is a group of inflammatory conditions of the colon and small intestine, Crohn's disease and ulcerative colitis being the principal types.[3] Crohn's disease affects the small intestine and large intestine, as well as the mouth, esophagus, stomach and the anus, whereas ulcerative colitis primarily affects the colon and the rectum.[4][5][6]
IBD also occurs in dogs and is thought to arise from a combination of host genetics, intestinal microenvironment, environmental components and the immune system. There is an ongoing discussion, however, that the term "chronic enteropathy" might be better to use than "inflammatory bowel disease" in dogs because it differs from IBD in humans in how the dogs respond to treatment. For example, many dogs respond to only dietary changes compared to humans with IBD, who often need immunosuppressive treatment. Some dogs may also need immunosuppressant or antibiotic treatment when dietary changes are not enough. After having excluded other diseases that can lead to vomiting, diarrhea, and abdominal pain in dogs, intestinal biopsies are often performed to investigate what kind of inflammation is occurring (lymphoplasmacytic, eosinophilic, or granulomatous). In dogs, low levels of cobalamin in the blood have been shown to be a risk factor for negative outcome.[7][8][9]
Signs and symptoms
| Crohn's disease | Ulcerative colitis | |
|---|---|---|
| Defecation | Often porridge-like,[10] sometimes steatorrhea | Often mucus-like and with blood[10] |
| Tenesmus | Less common[10] | More common[10] |
| Fever | Common[10] | Indicates severe disease[10] |
| Fistulae | Common[11] | Seldom |
| Weight loss | Often | More seldom |
In spite of Crohn's and UC being very different diseases, both may present with any of the following symptoms: abdominal pain, diarrhea, rectal bleeding, severe internal cramps/muscle spasms in the region of the pelvis and weight loss. Anemia is the most prevalent extraintestinal complication of inflammatory bowel disease.[12][13] Associated complaints or diseases include arthritis, pyoderma gangrenosum, primary sclerosing cholangitis, and non-thyroidal illness syndrome (NTIS).[14] Associations with deep vein thrombosis (DVT)[15] and bronchiolitis obliterans organizing pneumonia (BOOP) have also been reported.[16] Diagnosis is generally by assessment of inflammatory markers in stool followed by colonoscopy with biopsy of pathological lesions.
| Crohn's disease | Ulcerative colitis | |
|---|---|---|
| Terminal ileum involvement | Commonly | Seldom |
| Colon involvement | Usually | Always |
| Rectum involvement | Seldom | Usually (95%)[17] |
| Involvement around the anus | Common[11] | Seldom |
| Bile duct involvement | No increase in rate of primary sclerosing cholangitis | Higher rate[18] |
| Distribution of disease | Patchy areas of inflammation (skip lesions) | Continuous area of inflammation[17] |
| Endoscopy | Deep geographic and serpiginous (snake-like) ulcers | Continuous ulcer |
| Depth of inflammation | May be transmural, deep into tissues[11][5] | Shallow, mucosal |
| Stenosis | Common | Seldom |
| Granulomas on biopsy | May have non-necrotizing non-peri-intestinal crypt granulomas[11][19][20] | Non-peri-intestinal crypt granulomas not seen[21] |
Causes
| Crohn's disease | Ulcerative colitis | |
|---|---|---|
| Cytokine response | Associated with Th17[22] | Vaguely associated with Th2 |
IBD is a complex disease which arises as a result of the interaction of environmental and genetic factors leading to immunological responses and inflammation in the intestine.[4]
Diet
A 2022 study found that diets with increased intake of fruits and vegetables, reduction of processed meats and refined carbohydrates, and preference of water for hydration were associated with lower risk of active symptoms with IBD, although increased intake of fruits and vegetables alone did not reduce risk of symptoms with Crohn's disease.[23]
Dietary patterns are associated with a risk for ulcerative colitis. In particular, subjects who were in the highest tertile of the healthy dietary pattern had a 79% lower risk of ulcerative colitis.[24]
Gluten sensitivity is common in IBD and associated with having flareups. Gluten sensitivity was reported in 23.6 and 27.3% of Crohn's disease and ulcerative colitis patients, respectively.[25]
A diet high in protein, particularly animal protein, and/or high in sugar may be associated with increased risk of inflammatory bowel disease and relapses.[26][27]
Microbiota
As a result of microbial symbiosis and immunity, alterations in the gut microbiome may contribute to inflammatory gut diseases.[28] IBD-affected individuals have been found to have 30–50 percent reduced biodiversity of commensal bacteria, such as decreases in Bacillota (namely Lachnospiraceae) and Bacteroidota. Further evidence of the role of gut flora in the cause of inflammatory bowel disease is that IBD-affected individuals are more likely to have been prescribed antibiotics in the 2–5 year period before their diagnosis than unaffected individuals.[29] The enteral bacteria can be altered by environmental factors, such as concentrated milk fats (a common ingredient of processed foods and confectionery) or oral medications such as antibiotics and oral iron preparations.[30] The mucosal microbiota in the large intestine of IBD patients with active inflammation was found to be associated with pro-inflammatory changes to the host epigenome.[31] However, large international studies have failed to identify a single microbial biomarker of IBD indicating it's not driven by any single micro-organism.[32]
Breach of intestinal barrier
Loss of integrity of the intestinal epithelium plays a key pathogenic role in IBD.[33] Dysfunction of the innate immune system as a result of abnormal signaling through immune receptors called toll-like receptors (TLRs)—which activates an immune response to molecules that are broadly shared by multiple pathogens—contributes to acute and chronic inflammatory processes in IBD colitis and associated cancer.[34] Changes in the composition of the intestinal microbiota are an important environmental factor in the development of IBD. Detrimental changes in the intestinal microbiota induce an inappropriate (uncontrolled) immune response that results in damage to the intestinal epithelium. Breaches in this critical barrier (the intestinal epithelium) allow further infiltration of microbiota that, in turn, elicit further immune responses. IBD is a multifactorial disease that is nonetheless driven in part by an exaggerated immune response to gut microbiota that causes defects in epithelial barrier function.[35]
Oxidative stress and DNA damage
Pereira et al.[36] reviewed evidence from numerous studies indicating that oxidative stress and DNA damage likely have a role in the pathophysiology of IBD. Oxidative DNA damage as measured by 8-OHdG levels was found to be significantly increased in patients with IBD compared to control patients, and in inflamed mucosa compared with non-inflamed mucosa.[36]
Genetics
A genetic component to IBD has been recognized for over a century.[37] Research that has contributed to understanding of the genetics include studies of ethnic groups (e.g., Ashkenazi Jews, Irish), familial clustering, epidemiological studies, and twin studies. With the advent of molecular genetics, understanding of the genetic basis has expanded considerably, particularly in the past decade.[38] The first gene linked to IBD was NOD2 in 2001. Genome-wide association studies have since added to understanding of the genomics and pathogenesis of the disease. More than 200 single nucleotide polymorphisms (SNPs or "snips") are now known to be associated with susceptibility to IBD.[39] One of the largest genetic studies of IBD was published in 2012 .[40] The analysis explained more of the variance in Crohn's disease and ulcerative colitis than previously reported.[38] The results suggested that commensal microbiota are altered in such a way that they act as pathogens in inflammatory bowel diseases. Other studies show that mutations in IBD-associated genes might interfere with the cellular activity and interactions with the microbiome that promote normal immune responses.[41] Many studies identified that microRNAs dysregulation involved in IBD and to promote colorectal cancer.[42] By 2020, single-cell RNA sequencing analysis was launched by a small consortium using IBD patient biopsy material in a search for therapeutic targets.[43]
Diagnosis

The diagnosis is usually confirmed by biopsies on colonoscopy. Fecal calprotectin is useful as an initial investigation, which may suggest the possibility of IBD, as this test is sensitive but not specific for IBD.[44][45]
Differential diagnosis
Other diseases may cause an increased excretion of fecal calprotectin, such as infectious diarrhea, untreated coeliac disease, necrotizing enterocolitis, intestinal cystic fibrosis and neoplastic pediatric tumor cells.[46]
Conditions with similar symptoms as Crohn's disease includes intestinal tuberculosis, Behçet's disease, ulcerative colitis, nonsteroidal anti-inflammatory drug enteropathy, irritable bowel syndrome and coeliac disease.[47]
Conditions with similar symptoms as ulcerative colitis includes acute self-limiting colitis, amebic colitis, schistosomiasis, Crohn's disease, colon cancer, irritable bowel syndrome, intestinal tuberculosis and nonsteroidal anti-inflammatory drug enteropathy.[47]
Liver function tests are often elevated in inflammatory bowel disease, and are often mild and generally return spontaneously to normal levels.[48] The most relevant mechanisms of elevated liver functions tests in IBD are drug-induced hepatotoxicity and fatty liver.[48]
Classification
The chief types of inflammatory bowel disease are Crohn's disease and ulcerative colitis (UC). Inflammatory bowel diseases fall into the class of autoimmune diseases, in which the body's own immune system attacks elements of the digestive system.[49]
Accounting for fewer cases are other forms of IBD, which are not always classified as typical IBD:
- Microscopic colitis subdivided into collagenous colitis and lymphocytic colitis
- Diversion colitis
- Behçet's disease
- Early Onset IBD[50]
- Indeterminate colitis
No disease specific markers are currently known in the blood, enabling the reliable separation of Crohn's disease and ulcerative colitis patients. The way doctors can tell the difference between Crohn's disease and UC is the location and nature of the inflammatory changes. Crohn's can affect any part of the gastrointestinal tract, from mouth to anus (skip lesions), although a majority of the cases start in the terminal ileum. Ulcerative colitis, in contrast, is restricted to the colon and the rectum.[51] Microscopically, ulcerative colitis is restricted to the mucosa (epithelial lining of the gut), while Crohn's disease affects the full thickness of the bowel wall ("transmural lesions"). Lastly, Crohn's disease and ulcerative colitis present with extra-intestinal manifestations (such as liver problems, arthritis, skin manifestations and eye problems) in different proportions.
In 10–15% of cases,[52] a definitive diagnosis neither of Crohn's disease nor of ulcerative colitis can be made because of idiosyncrasies in the presentation. In this case, a diagnosis of indeterminate colitis may be made. Although a recognised definition, not all centres refer to this.
Treatment
| Crohn's disease | Ulcerative colitis | |
|---|---|---|
| Mesalazine | Less useful[53] | More useful[53] |
| Antibiotics | Effective in long-term[54] | Generally not useful[55] |
| Surgery | Often returns following removal of affected part | Usually cured by removal of colon |
Surgery
CD and UC are chronic inflammatory diseases, and are not medically curable.[56] However, ulcerative colitis can in most cases be cured by proctocolectomy, although this may not eliminate extra-intestinal symptoms. An ileostomy will collect feces in a bag. Alternatively, a pouch can be created from the small intestine; this serves as the rectum and prevents the need for a permanent ileostomy. Between one-quarter and one-half of patients with ileo-anal pouches do have to manage occasional or chronic pouchitis.[57]
Surgery cannot cure Crohn's disease but may be needed to treat complications such as abscesses, strictures or fistulae.[58] Severe cases may require surgery, such as bowel resection, strictureplasty or a temporary or permanent colostomy or ileostomy. In Crohn's disease, surgery involves removing the worst inflamed segments of the intestine and connecting the healthy regions, but unfortunately, it does not cure Crohn's or eliminate the disease. At some point after the first surgery, Crohn's disease can recur in the healthy parts of the intestine, usually at the resection site. (For example, if a patient with Crohn's disease has an ileocecal anastomosis, in which the caecum and terminal ileum are removed and the ileum is joined to the ascending colon, their Crohn's will nearly always flare-up near the anastomosis or in the rest of the ascending colon).
Medical therapies
Medical treatment of IBD is individualised to each patient.[56] The choice of which drugs to use and by which route to administer them (oral, rectal, injection, infusion) depends on factors including the type, distribution, and severity of the patient's disease, as well as other historical and biochemical prognostic factors, and patient preferences. For example, mesalazine is more useful in ulcerative colitis than in Crohn's disease.[53] Generally, depending on the level of severity, IBD may require immunosuppression to control the symptoms, with drugs such as prednisone, tumor necrosis factor inhibitors (TNF inhibitors), azathioprine, methotrexate, or 6-mercaptopurine.
Steroids, such as the glucocorticoid prednisone, are frequently used to control disease flares and were once acceptable as a maintenance drug. Biological therapy for inflammatory bowel disease, especially the TNF inhibitors, are used in people with more severe or resistant Crohn's disease and sometimes in ulcerative colitis.[59]
Treatment is usually started by administering drugs with high anti-inflammatory effects, such as prednisone. Once the inflammation is successfully controlled, another drug to keep the disease in remission, such as mesalazine in UC, is the main treatment. If further treatment is required, a combination of an immunosuppressive drug (such as azathioprine) with mesalazine (which may also have an anti-inflammatory effect) may be needed, depending on the patient. Controlled release budesonide is used for mild ileal Crohn's disease.[56]
Nutritional and dietetic therapies
Exclusive enteral nutrition is a first-line therapy in pediatric Crohn's disease with weaker data in adults.[60]: 331 [61] Evidence supporting exclusive enteral nutrition in ulcerative colitis is lacking.[60]: 333
Nutritional deficiencies play a prominent role in IBD. Malabsorption, diarrhea, and GI blood loss are common features of IBD. Deficiencies of B vitamins, fat-soluble vitamins, essential fatty acids, and key minerals such as magnesium, zinc, and selenium are extremely common and benefit from replacement therapy. Dietary interventions, including certain exclusion diets like the specific carbohydrate diet (SCD) can be beneficial for symptom management.[62] Dietary fiber interventions, such as psyillium supplementation (a mixture of soluble and insoluble fibers), may relieve symptoms as well as induce/maintain remission by altering the microbiome composition of the GI tract, thereby improving regulation of immune function, reducing inflammation, and helping to restore the intestinal mucosal lining.[63]
Anaemia is commonly present in both ulcerative colitis and Crohn's disease. Due to raised levels of inflammatory cytokines which lead to the increased expression of hepcidin, parenteral iron is the preferred treatment option as it bypasses the gastrointestinal system, has lower incidence of adverse events and enables quicker treatment. Hepcidin itself is also an anti-inflammatory agent. In the murine model very low levels of iron restrict hepcidin synthesis, worsening the inflammation that is present.[64] Enteral nutrition has been found to be efficient to improve hemoglobin level in patients with inflammatory bowel disease, especially combined with erythropoietin.[65]
Microbiome
There is preliminary evidence of an infectious contribution to inflammatory bowel disease in some patients that may benefit from antibiotic therapy, such as with rifaximin.[66] The evidence for a benefit of rifaximin is mostly limited to Crohn's disease with less convincing evidence supporting use in ulcerative colitis.[67][68]
Fecal microbiota transplant is a relatively new treatment option for IBD which has attracted attention since 2010.[69][70] Some preliminary studies have suggested benefits similar to those in Clostridium difficile infection but a review of use in IBD shows that FMT is safe, but of variable efficacy. A 2014 review stated that more randomized controlled trials were needed.[70]
Alternative medicine
Complementary and alternative medicine approaches have been used in inflammatory bowel disorders.[71] Evidence from controlled studies of these therapies has been reviewed; risk of bias was quite heterogeneous. The best supportive evidence was found for herbal therapy, with Plantago ovata and curcumin in UC maintenance therapy, wormwood in CD, mind/body therapy and self-intervention in UC, and acupuncture in UC and CD.[72]
Novel approaches
Stem cell therapy is undergoing research as a possible treatment for IBD. A review of studies suggests a promising role, although there are substantial challenges, including cost and characterization of effects, which limit the current use in clinical practice.[73]
Psychological interventions
Currently, there is no evidence to recommend psychological treatment, such as psychotherapy, stress management and patient's education, to all adults with IBD in general.[74] These treatments had no effect on quality of life, emotional well-being and disease activity.[74] The need for these approaches should be individually assessed and further researched to identify subgroups and determine type of therapy that may benefit individuals with IBD.[74] In adolescents population such treatments may be beneficial on quality of life and depression, although only short-term effects have been found, which also imposes the need for further research.[74]
Prognosis
| Crohn's disease | Ulcerative colitis | ||
|---|---|---|---|
| Nutrient deficiency | Higher risk | ||
| Colon cancer risk | Slight | Considerable | |
| Percent of people with extraintestinal complications[75][76][77] | |||
| Iritis/uveitis | Females | 2.2% | 3.2% |
| Males | 1.3% | 0.9% | |
| Primary sclerosing cholangitis | Females | 0.3% | 1% |
| Males | 0.4% | 3% | |
| Ankylosing spondylitis | Females | 0.7% | 0.8% |
| Males | 2.7% | 1.5% | |
| Pyoderma gangrenosum | Females | 1.2% | 0.8% |
| Males | 1.3% | 0.7% | |
| Erythema nodosum | Females | 1.9% | 2% |
| Males | 0.6% | 0.7% | |
While IBD can limit quality of life because of pain, vomiting, and diarrhea, it is rarely fatal on its own. Fatalities due to complications such as toxic megacolon, bowel perforation and surgical complications are also rare.. Fatigue is a common symptom of IBD and can be a burden.[78]
Around one-third of individuals with IBD experience persistent gastrointestinal symptoms similar to irritable bowel syndrome (IBS) in the absence of objective evidence of disease activity.[79] Despite enduring the side-effects of long-term therapies, this cohort has a quality of life that is not significantly different to that of individuals with uncontrolled, objectively active disease, and escalation of therapy to biological agents is typically ineffective in resolving their symptoms.[80] The cause of these IBS-like symptoms is unclear, but it has been suggested that changes in the gut-brain axis, epithelial barrier dysfunction, and the gut flora may be partially responsible.[81]
While patients of IBD do have an increased risk of colorectal cancer, this is usually caught much earlier than the general population in routine surveillance of the colon by colonoscopy, and therefore patients are much more likely to survive.
New evidence suggests that patients with IBD may have an elevated risk of endothelial dysfunction and coronary artery disease.[82][83]
The goal of treatment is toward achieving remission, after which the patient is usually switched to a lighter drug with fewer potential side effects. Every so often, an acute resurgence of the original symptoms may appear; this is known as a "flare-up". Depending on the circumstances, it may go away on its own or require medication. The time between flare-ups may be anywhere from weeks to years, and varies wildly between patients – a few have never experienced a flare-up.
Life with IBD can be challenging; however, many sufferers lead relatively normal lives. IBD carries a psychological burden due to stigmatization of being diagnosed, leading to high levels of anxiety, depression, and a general reduction in the quality of life for sufferers.[84][85] Although living with IBD can be difficult, there are numerous resources available to help families navigate the ins and out of IBD, such as the Crohn's and Colitis Foundation of America (CCFA).
Epidemiology
IBD resulted in a global total of 51,000 deaths in 2013 and 55,000 deaths in 1990.[86] The increased incidence of IBD since World War 2 has been correlated to the increase in meat consumption worldwide, supporting the claim that animal protein intake is associated with IBD.[87] However, there are many environmental risk factors that have been linked to the increased and decreased risk of IBD, such as smoking, air pollution and greenspace, urbanization and Westernization.[88] Inflammatory bowel diseases are increasing in Europe.[89] Incidence and prevalence of IBD has risen steadily for the last decades in Asia, which could be related changes in diet and other environmental factors.[90]
Around 0.8% of people in the UK have IBD.[91] Similarly, around 270,000 (0.7%) of people in Canada have IBD,[92] with that number expected to rise to 400,000 (1%) by 2030.[93]
Research
The following treatment strategies are not used routinely, but appear promising in some forms of inflammatory bowel disease.
Initial reports[94] suggest that "helminthic therapy" may not only prevent but even control IBD: a drink with roughly 2,500 ova of the Trichuris suis helminth taken twice monthly decreased symptoms markedly in many patients. It is even speculated that an effective "immunization" procedure could be developed—by ingesting the cocktail at an early age.
Prebiotics and probiotics are focusing increasing interest as treatments for IBD. Currently, there is evidence to support the use of certain probiotics in addition to standard treatments in people with ulcerative colitis but there is no sufficient data to recommend probiotics in people suffering Crohn's disease. Further research is required to identify specific probiotic strains or their combinations and prebiotic substances for therapies of intestinal inflammation.[95] Currently, the probiotic strain, frequency, dose and duration of the probiotic therapy are not established.[96] In severely ill people with IBD there is a risk of the passage of viable bacteria from the gastrointestinal tract to the internal organs (bacterial translocation) and subsequent bacteremia, which can cause serious adverse health consequences.[96] Live bacteria might not be essential because of beneficial effects of probiotics seems to be mediated by their DNA and by secreted soluble factors, and their therapeutic effects may be obtained by systemic administration rather than oral administration.[96][97]
In 2005 New Scientist published a joint study by Bristol University and the University of Bath on the apparent healing power of cannabis on IBD. Reports that cannabis eased IBD symptoms indicated the possible existence of cannabinoid receptors in the intestinal lining, which respond to molecules in the plant-derived chemicals. CB1 cannabinoid receptors – which are known to be present in the brain – exist in the endothelial cells which line the gut, it is thought that they are involved in repairing the lining of the gut when damaged.[98]
The team deliberately damaged the cells to cause inflammation of the gut lining and then added synthetically produced cannabinoids; the result was that gut started to heal: the broken cells were repaired and brought back closer together to mend the tears. It is believed that in a healthy gut, natural endogenous cannabinoids are released from endothelial cells when they are injured, which then bind to the CB1 receptors. The process appears to set off a wound-healing reaction, and when people use cannabis, the cannabinoids bind to these receptors in the same way.[98]
Previous studies have shown that CB1 receptors located on the nerve cells in the gut respond to cannabinoids by slowing gut motility, therefore reducing the painful muscle contractions associated with diarrhea. CB2, another cannabinoid receptor predominantly expressed by immune cells, was detected in the gut of IBD sufferers at a higher concentration. These receptors, which also respond to chemicals in cannabis, appear to be associated with apoptosis – programmed cell death – and may have a role in suppressing the overactive immune system and reducing inflammation by mopping up excess cells.[98]
Activation of the endocannabinoid system was found efficient in ameliorating colitis and increasing the survival rate of mice, and reducing remote organ changes induced by colitis, further suggest that modulation of this system is a potential therapeutic approach for IBDs and the associated remote organ lesions.[99]
Alicaforsen is a first generation antisense oligodeoxynucleotide designed to bind specifically to the human ICAM-1 messenger RNA through Watson-Crick base pair interactions in order to subdue expression of ICAM-1.[100] ICAM-1 propagates an inflammatory response promoting the extravasation and activation of leukocytes (white blood cells) into inflamed tissue.[100] Increased expression of ICAM-1 has been observed within the inflamed intestinal mucosa of ulcerative colitis, pouchitis and Crohn's sufferers where ICAM-1 over production correlated with disease activity.[101] This suggests that ICAM-1 is a potential therapeutic target in the treatment of these diseases.[102][103]
Cannabinoid CB2 receptor agonists are found to decrease the induction of ICAM-1 and VCAM-1 surface expression in human brain tissues and primary human brain endothelial cells (BMVEC) exposed to various pro-inflammatory mediators.[104]
In 2014, an alliance among the Broad Institute, Amgen and Massachusetts General Hospital formed with the intention to "collect and analyze patient DNA samples to identify and further validate genetic targets."[105]
In 2015, a meta-analysis on 938 IBD patients and 953 controls, IBD was significantly associated with having higher odds of vitamin D deficiency.[106]
Gram-positive bacteria present in the lumen could be associated with extending the time of relapse for ulcerative colitis.[107]
Bidirectional pathways between depression and IBD have been suggested [108] and psychological processes have been demonstrated to influence self-perceived physical and psychological health over time.[109] IBD-disease activity may impact quality of life and over time may significantly affect individual's mental well-being, which may be related to the increased risk to develop anxiety and/or depression.[108][110][111] On the other hand, psychological distress may also influence IBD activity.[112]
Higher rates of anxiety and depression are observed among those with IBD compared to healthy individuals, which correlated with disease severity.[110][112] Moreover, anxiety and depression rates increase during active disease compared with inactive phases.[112]
See also
- Inflammatory bowel disease-22
- World Inflammatory Bowel Disease Day
References
- ↑ GBD 2015 Disease and Injury Incidence and Prevalence Collaborators (October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
- ↑ GBD 2015 Disease and Injury Incidence and Prevalence Collaborators (October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1459–1544. doi:10.1016/S0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
- ↑ Talley N (2018). Clinical examination : a systematic guide to physical diagnosis. Chatswood, N.S.W: Elsevier Australia. p. 227. ISBN 978-0-7295-4259-3. OCLC 988941211.
- 1 2 Baumgart DC, Carding SR (May 2007). "Inflammatory bowel disease: cause and immunobiology". Lancet. 369 (9573): 1627–40. doi:10.1016/S0140-6736(07)60750-8. PMID 17499605. S2CID 13544348.
- 1 2 Baumgart DC, Sandborn WJ (May 2007). "Inflammatory bowel disease: clinical aspects and established and evolving therapies". The Lancet. 369 (9573): 1641–57. doi:10.1016/S0140-6736(07)60751-X. PMID 17499606. Retrieved 2009-11-04.
- ↑ Xavier RJ, Podolsky DK (July 2007). "Unravelling the pathogenesis of inflammatory bowel disease". Nature. 448 (7152): 427–34. Bibcode:2007Natur.448..427X. doi:10.1038/nature06005. PMID 17653185. S2CID 4337332.
- ↑ Dandrieux JR (November 2016). "Inflammatory bowel disease versus chronic enteropathy in dogs: are they one and the same?". The Journal of Small Animal Practice. 57 (11): 589–599. doi:10.1111/jsap.12588. hdl:11343/291847. PMID 27747868.
- ↑ Allenspach K, Wieland B, Gröne A, Gaschen F (July 2007). "Chronic enteropathies in dogs: evaluation of risk factors for negative outcome". Journal of Veterinary Internal Medicine. 21 (4): 700–8. doi:10.1111/j.1939-1676.2007.tb03011.x. PMID 17708389.
- ↑ Simpson KW, Jergens AE (March 2011). "Pitfalls and progress in the diagnosis and management of canine inflammatory bowel disease". The Veterinary Clinics of North America. Small Animal Practice. Chronic Intestinal Diseases of Dogs and Cats. 41 (2): 381–98. doi:10.1016/j.cvsm.2011.02.003. PMID 21486642.
- 1 2 3 4 5 6 internetmedicin.se > Inflammatorisk tarmsjukdom, kronisk, IBD By Robert Löfberg. Retrieved Oct 2010 Translate.
- 1 2 3 4 Hanauer SB, Sandborn W (2001-03-01). "Management of Crohn's disease in adults" (PDF). American Journal of Gastroenterology. 96 (3): 635–43. doi:10.1111/j.1572-0241.2001.03671.x. PMID 11280528. Retrieved 2009-11-07.
- ↑ Wang GF, Ren JA, Liu S, Chen J, Gu GS, Wang XB, et al. (July 2012). "Clinical characteristics of non-perianal fistulating Crohn's disease in China: a single-center experience of 184 cases". Chinese Medical Journal. 125 (14): 2405–10. PMID 22882911.
- ↑ Stein J, Hartmann F, Dignass AU (November 2010). "Diagnosis and management of iron deficiency anemia in patients with IBD". Nature Reviews. Gastroenterology & Hepatology. 7 (11): 599–610. doi:10.1038/nrgastro.2010.151. PMID 20924367. S2CID 25341683.
- ↑ Liu S, Ren J, Zhao Y, Han G, Hong Z, Yan D, et al. (February 2013). "Nonthyroidal illness syndrome: is it far away from Crohn's disease?". Journal of Clinical Gastroenterology. 47 (2): 153–9. doi:10.1097/MCG.0b013e318254ea8a. PMID 22874844. S2CID 35344744.
- ↑ Warner J (February 22, 2011). Martin LJ (ed.). "Inflammatory Bowel Disease Health Center". WebMD. Archived from the original on 20 October 2014. Retrieved 14 October 2014.
- ↑ Lu DG, Ji XQ, Liu X, Li HJ, Zhang CQ (January 2014). "Pulmonary manifestations of Crohn's disease". World Journal of Gastroenterology. 20 (1): 133–41. doi:10.3748/wjg.v20.i1.133. PMC 3886002. PMID 24415866.
- 1 2 Rubin, DT; Ananthakrishnan, AN; Siegel, CA; Sauer, BG; Long, MD (March 2019). "ACG Clinical Guideline: Ulcerative Colitis in Adults". The American journal of gastroenterology. 114 (3): 384–413. doi:10.14309/ajg.0000000000000152. PMID 30840605.
- ↑ Broomé U, Bergquist A (February 2006). "Primary sclerosing cholangitis, inflammatory bowel disease, and colon cancer". Seminars in Liver Disease. 26 (1): 31–41. doi:10.1055/s-2006-933561. PMID 16496231.
- ↑ Shepherd NA (August 2002). "Granulomas in the diagnosis of intestinal Crohn's disease: a myth exploded?". Histopathology. 41 (2): 166–8. doi:10.1046/j.1365-2559.2002.01441.x. PMID 12147095.
- ↑ Mahadeva U, Martin JP, Patel NK, Price AB (July 2002). "Granulomatous ulcerative colitis: a re-appraisal of the mucosal granuloma in the distinction of Crohn's disease from ulcerative colitis". Histopathology. 41 (1): 50–5. doi:10.1046/j.1365-2559.2002.01416.x. PMID 12121237.
- ↑ DeRoche, TC; Xiao, SY; Liu, X (August 2014). "Histological evaluation in ulcerative colitis". Gastroenterology report. 2 (3): 178–92. doi:10.1093/gastro/gou031. PMID 24942757.
- ↑ Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA (2007). "Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice". Gastroenterology. 132 (7): 2359–70. doi:10.1053/j.gastro.2007.03.104. PMID 17570211.
- ↑ Limketkai, Berkeley N.; Hamideh, Mohamed; Shah, Rishabh; Sauk, Jenny S.; Jaffe, Nancee (2022-01-29). "Dietary Patterns and Their Association With Symptoms Activity in Inflammatory Bowel Diseases". Inflammatory Bowel Diseases: izab335. doi:10.1093/ibd/izab335. ISSN 1536-4844. PMID 35092268. Archived from the original on 2022-03-26. Retrieved 2022-04-12.
- ↑ Rashvand S, Behrooz M, Samsamikor M, Jacobson K, Hekmatdoost A (June 2018). "Dietary patterns and risk of ulcerative colitis: a case-control study". Journal of Human Nutrition and Dietetics. 31 (3): 408–412. doi:10.1111/jhn.12544. PMID 29468761. S2CID 3452004.
- ↑ Limketkai BN, Sepulveda R, Hing T, Shah ND, Choe M, Limsui D, Shah S (February 2018). "Prevalence and factors associated with gluten sensitivity in inflammatory bowel disease". Scandinavian Journal of Gastroenterology. 53 (2): 147–151. doi:10.1080/00365521.2017.1409364. PMID 29216767. S2CID 4119392.
- ↑ Andersen V, Olsen A, Carbonnel F, Tjønneland A, Vogel U (March 2012). "Diet and risk of inflammatory bowel disease". Digestive and Liver Disease. 44 (3): 185–94. doi:10.1016/j.dld.2011.10.001. PMID 22055893.
- ↑ Barron M (2021-01-11). "Sugar may trigger inflammatory bowel disease by breaking down gut mucus". Massive Science. Archived from the original on 2021-01-13. Retrieved 2021-01-15.
- ↑ Mukhopadhya I, Hansen R, El-Omar EM, Hold GL (February 2012). "IBD-what role do Proteobacteria play?". Nature Reviews. Gastroenterology & Hepatology. 9 (4): 219–30. doi:10.1038/nrgastro.2012.14. PMID 22349170. S2CID 24538712.
- ↑ Aroniadis OC, Brandt LJ (January 2013). "Fecal microbiota transplantation: past, present and future". Current Opinion in Gastroenterology. 29 (1): 79–84. doi:10.1097/MOG.0b013e32835a4b3e. PMID 23041678. S2CID 39943619.
- ↑ Kotanko P, Carter M, Levin NW (August 2006). "Intestinal bacterial microflora--a potential source of chronic inflammation in patients with chronic kidney disease". Nephrology, Dialysis, Transplantation. 21 (8): 2057–60. doi:10.1093/ndt/gfl281. PMID 16762961.
- ↑ Ryan, F. J.; Ahern, A. M.; Fitzgerald, R. S.; Laserna-Mendieta, E. J.; Power, E. M.; Clooney, A. G.; O'Donoghue, K. W.; McMurdie, P. J.; Iwai, S.; Crits-Christoph, A.; Sheehan, D. (2020-03-23). "Colonic microbiota is associated with inflammation and host epigenomic alterations in inflammatory bowel disease". Nature Communications. 11 (1): 1512. Bibcode:2020NatCo..11.1512R. doi:10.1038/s41467-020-15342-5. ISSN 2041-1723. PMC 7089947. PMID 32251296.
- ↑ Clooney, Adam G.; Eckenberger, Julia; Laserna-Mendieta, Emilio; Sexton, Kathryn A.; Bernstein, Matthew T.; Vagianos, Kathy; Sargent, Michael; Ryan, Feargal J.; Moran, Carthage; Sheehan, Donal; Sleator, Roy D. (March 2021). "Ranking microbiome variance in inflammatory bowel disease: a large longitudinal intercontinental study". Gut. 70 (3): 499–510. doi:10.1136/gutjnl-2020-321106. ISSN 1468-3288. PMC 7873428. PMID 32536605.
- ↑ Maloy KJ, Powrie F (June 2011). "Intestinal homeostasis and its breakdown in inflammatory bowel disease". Nature. 474 (7351): 298–306. doi:10.1038/nature10208. PMID 21677746. S2CID 205225483. Archived from the original on 2019-10-29. Retrieved 2022-04-12.
- ↑ Cario E (September 2010). "Toll-like receptors in inflammatory bowel diseases: a decade later". Inflammatory Bowel Diseases. 16 (9): 1583–97. doi:10.1002/ibd.21282. PMC 2958454. PMID 20803699.
- ↑ Coskun M (2014-08-25). "Intestinal epithelium in inflammatory bowel disease". Frontiers in Medicine. 1: 24. doi:10.3389/fmed.2014.00024. PMC 4292184. PMID 25593900.
- 1 2 Pereira, Cristiana; Grácio, Daniela; Teixeira, João P.; Magro, Fernando (October 2015). "Oxidative Stress and DNA Damage: Implications in Inflammatory Bowel Disease". Inflammatory Bowel Diseases. 21 (10): 2403–2417. doi:10.1097/MIB.0000000000000506. PMID 26193347. S2CID 8068289.
- ↑ Ek WE, D'Amato M, Halfvarson J (2014). "The history of genetics in inflammatory bowel disease". Annals of Gastroenterology. 27 (4): 294–303. PMC 4188925. PMID 25331623.
- 1 2 Liu TC, Stappenbeck TS (May 2016). "Genetics and Pathogenesis of Inflammatory Bowel Disease". Annual Review of Pathology. 11: 127–48. doi:10.1146/annurev-pathol-012615-044152. PMC 3204665. PMID 26907531.
- ↑ Ye BD, McGovern DP (October 2016). "Genetic variation in IBD: progress, clues to pathogenesis and possible clinical utility". Expert Review of Clinical Immunology. 12 (10): 1091–107. doi:10.1080/1744666X.2016.1184972. PMC 5083126. PMID 27156530.
- ↑ Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. (November 2012). "Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease". Nature. 491 (7422): 119–24. Bibcode:2012Natur.491..119.. doi:10.1038/nature11582. PMC 3491803. PMID 23128233.
- ↑ Chu H, Khosravi A, Kusumawardhani IP, Kwon AH, Vasconcelos AC, Cunha LD, et al. (May 2016). "Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease". Science. 352 (6289): 1116–20. Bibcode:2016Sci...352.1116C. doi:10.1126/science.aad9948. PMC 4996125. PMID 27230380.
- ↑ Bocchetti M, Ferraro MG, Ricciardiello F, Ottaiano A, Luce A, Cossu AM, et al. (April 2021). "The Role of microRNAs in Development of Colitis-Associated Colorectal Cancer". International Journal of Molecular Sciences. 22 (8): 3967. doi:10.3390/ijms22083967. PMC 8068787. PMID 33921348.
- ↑ Staff (1 July 2020). "Celsius Therapeutics Teams With Oxford, Cleveland Clinic, LMU on Single-Cell IBD Research". genomeweb. New York City: Crain Communications. Archived from the original on 2 July 2020. Retrieved 1 July 2020.
- ↑ Henderson P, Anderson NH, Wilson DC (May 2014). "The diagnostic accuracy of fecal calprotectin during the investigation of suspected pediatric inflammatory bowel disease: a systematic review and meta-analysis". The American Journal of Gastroenterology. 109 (5): 637–45. doi:10.1038/ajg.2013.131. PMID 23670113. S2CID 30604736.
- ↑ Waugh N, Cummins E, Royle P, Kandala NB, Shyangdan D, Arasaradnam R, et al. (November 2013). "Faecal calprotectin testing for differentiating amongst inflammatory and non-inflammatory bowel diseases: systematic review and economic evaluation". Health Technology Assessment. 17 (55): xv–xix, 1–211. doi:10.3310/hta17550. PMC 4781415. PMID 24286461. Archived from the original on 2022-05-07. Retrieved 2022-04-12.
- ↑ Vaos G, Kostakis ID, Zavras N, Chatzemichael A (2013). "The role of calprotectin in pediatric disease". BioMed Research International (Review). 2013: 542363. doi:10.1155/2013/542363. PMC 3794633. PMID 24175291.
- 1 2 "Inflammatory Bowel Disease" (PDF). World Gastroenterology Organization. August 2015. Archived (PDF) from the original on March 14, 2016. Retrieved Mar 13, 2016.
- 1 2 Cappello M, Randazzo C, Bravatà I, Licata A, Peralta S, Craxì A, Almasio PL (2014). "Liver Function Test Abnormalities in Patients with Inflammatory Bowel Diseases: A Hospital-based Survey". Clinical Medicine Insights. Gastroenterology. 7: 25–31. doi:10.4137/CGast.S13125. PMC 4069044. PMID 24966712.
- ↑ "IBD Facts". Archived from the original on 2013-02-12. Retrieved 2013-02-13.
- ↑ Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schäffer AA, Noyan F, et al. (November 2009). "Inflammatory bowel disease and mutations affecting the interleukin-10 receptor". The New England Journal of Medicine. 361 (21): 2033–2045. doi:10.1056/NEJMoa0907206. PMC 2787406. PMID 19890111.
- ↑ "Crohn's & Colitis Foundation of America". Archived from the original on 2016-07-27. Retrieved 2022-05-07.
- ↑ Guindi M, Riddell RH (December 2004). "Indeterminate colitis". Journal of Clinical Pathology. 57 (12): 1233–44. doi:10.1136/jcp.2003.015214. PMC 1770507. PMID 15563659.
- 1 2 3 Agabegi, Elizabeth D; Agabegi, Steven S. (2008). "Inflammatory bowel disease (IBD)". Step-Up to Medicine (Step-Up Series). Hagerstwon, MD: Lippincott Williams & Wilkins. pp. 152–156. ISBN 0-7817-7153-6.
{{cite book}}: Unknown parameter|name-list-format=ignored (help) - ↑ Feller M, Huwiler K, Schoepfer A, Shang A, Furrer H, Egger M (February 2010). "Long-term antibiotic treatment for Crohn's disease: systematic review and meta-analysis of placebo-controlled trials". Clinical Infectious Diseases. 50 (4): 473–80. doi:10.1086/649923. PMID 20067425.
- ↑ Prantera C, Scribano ML (July 2009). "Antibiotics and probiotics in inflammatory bowel disease: why, when, and how". Current Opinion in Gastroenterology. 25 (4): 329–33. doi:10.1097/MOG.0b013e32832b20bf. PMID 19444096.
- 1 2 3 Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, et al. (May 2011). "Guidelines for the management of inflammatory bowel disease in adults". Gut. 60 (5): 571–607. doi:10.1136/gut.2010.224154. PMID 21464096. S2CID 8269837. Archived from the original on 2022-05-07. Retrieved 2022-04-12.
- ↑ "Pouchitis: Symptoms & Causes". Mayo Clinic. 21 December 2018. Archived from the original on 31 October 2013. Retrieved 12 April 2022.
- ↑ Karimuddin A, Gilles G. "Surgery for Abdominal/Intestinal Crohn's Disease". Trusted Therapies. Trusted Therapies. Archived from the original on 23 July 2016. Retrieved 19 May 2015.
- ↑ D'Haens GR, Panaccione R, Higgins PD, Vermeire S, Gassull M, Chowers Y, et al. (February 2011). "The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn's and Colitis Organization: when to start, when to stop, which drug to choose, and how to predict response?". The American Journal of Gastroenterology. 106 (2): 199–212, quiz 213. doi:10.1038/ajg.2010.392. PMID 21045814. S2CID 24401720.
- 1 2 Forbes A, Escher J, Hébuterne X, Kłęk S, Krznaric Z, Schneider S, et al. (April 2017). "ESPEN guideline: Clinical nutrition in inflammatory bowel disease". Clinical Nutrition. 36 (2): 321–347. doi:10.1016/j.clnu.2016.12.027. PMID 28131521.
- ↑ Ashton JJ, Gavin J, Beattie RM (February 2019). "Exclusive enteral nutrition in Crohn's disease: Evidence and practicalities". Clinical Nutrition. 38 (1): 80–89. doi:10.1016/j.clnu.2018.01.020. PMID 29398336. S2CID 25135919. Archived from the original on 2022-01-13. Retrieved 2022-04-12.
- ↑ Charlebois A, Rosenfeld G, Bressler B (June 2016). "The Impact of Dietary Interventions on the Symptoms of Inflammatory Bowel Disease: A Systematic Review". Critical Reviews in Food Science and Nutrition. 56 (8): 1370–8. doi:10.1080/10408398.2012.760515. PMID 25569442. S2CID 267557.
- ↑ Wong C, Harris PJ, Ferguson LR (June 2016). "Potential Benefits of Dietary Fibre Intervention in Inflammatory Bowel Disease". International Journal of Molecular Sciences. 17 (6): 919. doi:10.3390/ijms17060919. PMC 4926452. PMID 27314323.
- ↑ Pagani A, Nai A, Corna G, Bosurgi L, Rovere-Querini P, Camaschella C, Silvestri L (July 2011). "Low hepcidin accounts for the proinflammatory status associated with iron deficiency". Blood. 118 (3): 736–46. doi:10.1182/blood-2011-02-337212. PMID 21628413.
- ↑ Liu S, Ren J, Hong Z, Yan D, Gu G, Han G, et al. (February 2013). "Efficacy of erythropoietin combined with enteral nutrition for the treatment of anemia in Crohn's disease: a prospective cohort study". Nutrition in Clinical Practice. 28 (1): 120–7. doi:10.1177/0884533612462744. PMID 23064018.
- ↑ Lopetuso LR, Napoli M, Rizzatti G, Gasbarrini A (June 2018). "The intriguing role of Rifaximin in gut barrier chronic inflammation and in the treatment of Crohn's disease". Expert Opinion on Investigational Drugs. 27 (6): 543–551. doi:10.1080/13543784.2018.1483333. PMID 29865875. S2CID 46928303.
- ↑ Scribano ML (2015). "Role of Rifaximin in Inflammatory Bowel Disease Treatment". Mini Reviews in Medicinal Chemistry. 16 (3): 225–9. doi:10.2174/1389557515666150722104230. PMID 26202194.
- ↑ Sartor RB (January 2016). "Review article: the potential mechanisms of action of rifaximin in the management of inflammatory bowel diseases". Alimentary Pharmacology & Therapeutics. 43 (Suppl 1): 27–36. doi:10.1111/apt.13436. PMID 26618923. S2CID 26119818.
- ↑ Sunkara T, Rawla P, Ofosu A, Gaduputi V (2018). "Fecal microbiota transplant - a new frontier in inflammatory bowel disease". Journal of Inflammation Research. 11: 321–328. doi:10.2147/JIR.S176190. PMC 6124474. PMID 30214266.
- 1 2 Colman RJ, Rubin DT (December 2014). "Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis". Journal of Crohn's & Colitis. 8 (12): 1569–81. doi:10.1016/j.crohns.2014.08.006. PMC 4296742. PMID 25223604.
- ↑ Gilardi D, Fiorino G, Genua M, Allocca M, Danese S (September 2014). "Complementary and alternative medicine in inflammatory bowel diseases: what is the future in the field of herbal medicine?". Expert Review of Gastroenterology & Hepatology. 8 (7): 835–46. doi:10.1586/17474124.2014.917954. PMID 24813226. S2CID 207205293.
- ↑ Langhorst J, Wulfert H, Lauche R, Klose P, Cramer H, Dobos GJ, Korzenik J (January 2015). "Systematic review of complementary and alternative medicine treatments in inflammatory bowel diseases". Journal of Crohn's & Colitis. 9 (1): 86–106. doi:10.1093/ecco-jcc/jju007. PMID 25518050.
- ↑ Dave M, Mehta K, Luther J, Baruah A, Dietz AB, Faubion WA (November 2015). "Mesenchymal Stem Cell Therapy for Inflammatory Bowel Disease: A Systematic Review and Meta-analysis". Inflammatory Bowel Diseases. 21 (11): 2696–707. doi:10.1097/MIB.0000000000000543. PMC 4615553. PMID 26230863.
- 1 2 3 4 Timmer, Antje; Preiss, Jan C; Motschall, Edith; Rücker, Gerta; Jantschek, Günther; Moser, Gabriele (2011-02-15). Cochrane IBD Group (ed.). "Psychological interventions for treatment of inflammatory bowel disease". Cochrane Database of Systematic Reviews (2): CD006913. doi:10.1002/14651858.CD006913.pub2. PMID 21328288. Archived from the original on 2022-05-07. Retrieved 2022-04-12.
- ↑ Greenstein AJ, Janowitz HD, Sachar DB (September 1976). "The extra-intestinal complications of Crohn's disease and ulcerative colitis: a study of 700 patients". Medicine (Baltimore). 55 (5): 401–12. doi:10.1097/00005792-197609000-00004. PMID 957999.
- ↑ Bernstein, Charles N; Blanchard, James F; Rawsthorne, Patricia; Yu, Nancy (April 2001). "The Prevalence of Extraintestinal Diseases in Inflammatory Bowel Disease: A Population-Based Study:". American Journal of Gastroenterology. 96 (4): 1116–1122. doi:10.1111/j.1572-0241.2001.03756.x. PMID 11316157.
- ↑ Harbord, Marcus; Annese, Vito; Vavricka, Stephan R.; Allez, Matthieu; Barreiro-de Acosta, Manuel; Boberg, Kirsten Muri; Burisch, Johan; De Vos, Martine; De Vries, Anne-Marie; Dick, Andrew D.; Juillerat, Pascal; Karlsen, Tom H.; Koutroubakis, Ioannis; Lakatos, Peter L.; Orchard, Tim; Papay, Pavol; Raine, Tim; Reinshagen, Max; Thaci, Diamant; Tilg, Herbert; Carbonnel, Franck (1 March 2016). "The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease". Journal of Crohn's and Colitis. 10 (3): 239–254. doi:10.1093/ecco-jcc/jjv213. PMID 26614685.
- ↑ Farrell D, Artom M, Czuber-Dochan W, Jelsness-Jørgensen LP, Norton C, Savage E (April 2020). "Interventions for fatigue in inflammatory bowel disease". The Cochrane Database of Systematic Reviews. 2020 (4): CD012005. doi:10.1002/14651858.CD012005.pub2. PMC 7161727. PMID 32297974.
- ↑ Halpin SJ, Ford AC (October 2012). "Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis". The American Journal of Gastroenterology. 107 (10): 1474–82. doi:10.1038/ajg.2012.260. PMID 22929759. S2CID 11007309.
- ↑ Gracie DJ, Williams CJ, Sood R, Mumtaz S, Bholah MH, Hamlin PJ, Ford AC (March 2017). "Negative Effects on Psychological Health and Quality of Life of Genuine Irritable Bowel Syndrome-type Symptoms in Patients With Inflammatory Bowel Disease". Clinical Gastroenterology and Hepatology. 15 (3): 376–384.e5. doi:10.1016/j.cgh.2016.05.012. PMID 27189912.
- ↑ Abdul Rani R, Raja Ali RA, Lee YY (October 2016). "Irritable bowel syndrome and inflammatory bowel disease overlap syndrome: pieces of the puzzle are falling into place". Intestinal Research. 14 (4): 297–304. doi:10.5217/ir.2016.14.4.297. PMC 5083258. PMID 27799880.
- ↑ Gandhi S, Narula N, Marshall JK, Farkouh M (October 2012). "Are patients with inflammatory bowel disease at increased risk of coronary artery disease?". The American Journal of Medicine. 125 (10): 956–62. doi:10.1016/j.amjmed.2012.03.015. PMID 22840916.
- ↑ Roifman I, Sun YC, Fedwick JP, Panaccione R, Buret AG, Liu H, et al. (February 2009). "Evidence of endothelial dysfunction in patients with inflammatory bowel disease". Clinical Gastroenterology and Hepatology. 7 (2): 175–82. doi:10.1016/j.cgh.2008.10.021. PMID 19121648. Archived from the original on 2022-05-07. Retrieved 2022-04-12.
- ↑ Kemp K, Griffiths J, Lovell K. Understanding the health and social care needs ofpeople living with IBD: a meta-synthesis of the evidence. World J Gastroenterol2012;18:6240–9.
- ↑ Borghi L., Poli S., Furfaro F., Allocca M., Vegni E.A.M. Psychological Challenges for Patients with Inflammatory Bowel Disease during the COVID-19 Pandemic. Psychosom. Med.. 2021;83(4):397-398. doi:10.1097/PSY.0000000000000888
- ↑ GBD 2013 Mortality Causes of Death Collaborators (January 2015). "Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013". Lancet. 385 (9963): 117–71. doi:10.1016/S0140-6736(14)61682-2. PMC 4340604. PMID 25530442.
- ↑ Jantchou P, Morois S, Clavel-Chapelon F, Boutron-Ruault MC, Carbonnel F (October 2010). "Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study". The American Journal of Gastroenterology. 105 (10): 2195–201. doi:10.1038/ajg.2010.192. PMID 20461067. S2CID 13160121.
- ↑ Kaplan GG, Bernstein CN, Coward S, Bitton A, Murthy SK, Nguyen GC, Lee K, Cooke-Lauder J, Benchimol EI (November 2018). "The Impact of Inflammatory Bowel Disease in Canada 2018: Epidemiology". Journal of the Canadian Association of Gastroenterology. 2 (Suppl 1): S6–S16. doi:10.1093/jcag/gwy054. PMC 6512243. PMID 31294381.
- ↑ Burisch J, Jess T, Martinato M, Lakatos PL (May 2013). "The burden of inflammatory bowel disease in Europe". Journal of Crohn's & Colitis. 7 (4): 322–37. doi:10.1016/j.crohns.2013.01.010. PMID 23395397.
- ↑ Park J, Cheon JH (February 2021). "Incidence and Prevalence of Inflammatory Bowel Disease across Asia". Yonsei Medical Journal. 62 (2): 99–108. doi:10.3349/ymj.2021.62.2.99. PMC 7859683. PMID 33527789.
- ↑ British Society of Gastroenterology https://www.bsg.org.uk/covid-19-advice/bsg-advice-for-management-of-inflammatory-bowel-diseases-during-the-covid-19-pandemic/ Archived 2022-04-12 at the Wayback Machine
- ↑ Crohn's and Colitis Canada 2018 Impact of IBD in Canada Report. https://crohnsandcolitis.ca/About-Us/Resources-Publications/Impact-of-IBD-Report/ Archived 2022-04-07 at the Wayback Machine
- ↑ Coward S, Clement F, Benchimol EI, Bernstein CN, Avina-Zubieta JA, Bitton A, Carroll MW, Hazlewood G, Jacobson K, Jelinski S, Deardon R, Jones JL, Kuenzig ME, Leddin D, McBrien KA, Murthy SK, Nguyen GC, Otley AR, Panaccione R, Rezaie A, Rosenfeld G, Peña-Sánchez JN, Singh H, Targownik LE, Kaplan GG (May 2019). "Past and Future Burden of Inflammatory Bowel Diseases Based on Modeling of Population-Based Data". Gastroenterology. 156 (5): 1345–1353.e4. doi:10.1053/j.gastro.2019.01.002. PMID 30639677.
- ↑ Summers RW, Elliott DE, Qadir K, Urban JF, Thompson R, Weinstock JV (September 2003). "Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease". The American Journal of Gastroenterology. 98 (9): 2034–41. CiteSeerX 10.1.1.457.8633. doi:10.1111/j.1572-0241.2003.07660.x. PMID 14499784. S2CID 2605979.
- ↑ Ghouri YA, Richards DM, Rahimi EF, Krill JT, Jelinek KA, DuPont AW (Dec 9, 2014). "Systematic review of randomized controlled trials of probiotics, prebiotics, and synbiotics in inflammatory bowel disease". Clinical and Experimental Gastroenterology (Review). 7: 473–87. doi:10.2147/CEG.S27530. PMC 4266241. PMID 25525379.
- 1 2 3 Durchschein F, Petritsch W, Hammer HF (February 2016). "Diet therapy for inflammatory bowel diseases: The established and the new". World Journal of Gastroenterology (Review). 22 (7): 2179–94. doi:10.3748/wjg.v22.i7.2179. PMC 4734995. PMID 26900283.
- ↑ Dotan I, Rachmilewitz D (July 2005). "Probiotics in inflammatory bowel disease: possible mechanisms of action". Current Opinion in Gastroenterology (Review). 21 (4): 426–30. PMID 15930982.
- 1 2 3 Wright K, Rooney N, Feeney M, Tate J, Robertson D, Welham M, Ward S (August 2005). "Differential expression of cannabinoid receptors in the human colon: cannabinoids promote epithelial wound healing". Gastroenterology. 129 (2): 437–53. doi:10.1053/j.gastro.2005.05.026. PMID 16083701.
- ↑ Zhao X, Liang P, Liu J, Jiang H, Fan X, Chen G, Zhou C (December 2017). "Elevation of arachidonoylethanolamide levels by activation of the endocannabinoid system protects against colitis and ameliorates remote organ lesions in mice". Experimental and Therapeutic Medicine. 14 (6): 5664–5670. doi:10.3892/etm.2017.5222. PMC 5740744. PMID 29285108.
- 1 2 Bennett CF, Condon TP, Grimm S, Chan H, Chiang MY (April 1994). "Inhibition of endothelial cell adhesion molecule expression with antisense oligonucleotides". Journal of Immunology. 152 (7): 3530–40. PMID 7511650.
- ↑ Jones SC, Banks RE, Haidar A, Gearing AJ, Hemingway IK, Ibbotson SH, et al. (May 1995). "Adhesion molecules in inflammatory bowel disease". Gut. 36 (5): 724–30. doi:10.1136/gut.36.5.724. PMC 1382677. PMID 7541009.
- ↑ van Deventer SJ, Wedel MK, Baker BF, Xia S, Chuang E, Miner PB (May 2006). "A phase II dose ranging, double-blind, placebo-controlled study of alicaforsen enema in subjects with acute exacerbation of mild to moderate left-sided ulcerative colitis". Alimentary Pharmacology & Therapeutics. 23 (10): 1415–25. doi:10.1111/j.1365-2036.2006.02910.x. PMID 16669956. S2CID 31495688.
- ↑ Thomas S, Baumgart DC (February 2012). "Targeting leukocyte migration and adhesion in Crohn's disease and ulcerative colitis". Inflammopharmacology. 20 (1): 1–18. doi:10.1007/s10787-011-0104-6. PMID 22205271. S2CID 11663330.
- ↑ Ramirez SH, Haskó J, Skuba A, Fan S, Dykstra H, McCormick R, et al. (March 2012). "Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions". The Journal of Neuroscience. 32 (12): 4004–16. doi:10.1523/JNEUROSCI.4628-11.2012. PMC 3325902. PMID 22442067.
- ↑ "Amgen, MGH, Broad form IBD Therapeutics Initiative". News: Discovery & Development. Gen. Eng. Biotechnol. News (paper). Vol. 34, no. 4. p. 14.
- ↑ Del Pinto R, Pietropaoli D, Chandar AK, Ferri C, Cominelli F (November 2015). "Association Between Inflammatory Bowel Disease and Vitamin D Deficiency: A Systematic Review and Meta-analysis". Inflammatory Bowel Diseases. 21 (11): 2708–17. doi:10.1097/MIB.0000000000000546. PMC 4615394. PMID 26348447.
- ↑ Ghouri YA, Richards DM, Rahimi EF, Krill JT, Jelinek KA, DuPont AW (9 December 2014). "Systematic review of randomized controlled trials of probiotics, prebiotics, and synbiotics in inflammatory bowel disease". Clinical and Experimental Gastroenterology. 7: 473–87. doi:10.2147/CEG.S27530. PMC 4266241. PMID 25525379.
- 1 2 Keefer L, Kane SV (2017). "Considering the Bidirectional Pathways Between Depression and IBD: Recommendations for Comprehensive IBD Care". Gastroenterology & Hepatology. 13 (3): 164–169. PMC 5439135. PMID 28539843.
- ↑ Trindade IA, Ferreira C, Pinto-Gouveia J (February 2018). "The longitudinal effects of emotion regulation on physical and psychological health: A latent growth analysis exploring the role of cognitive fusion in inflammatory bowel disease". British Journal of Health Psychology. 23 (1): 171–185. doi:10.1111/bjhp.12280. hdl:10316/46833. PMID 28980414. S2CID 3375968.
- 1 2 Gao, Xin; Tang, Yu; Lei, Na; Luo, Ying; Chen, Pingrun; Liang, Chang; Duan, Shihao; Zhang, Yan (2021-01-14). "Symptoms of anxiety/depression is associated with more aggressive inflammatory bowel disease". Scientific Reports. 11 (1): 1440. Bibcode:2021NatSR..11.1440G. doi:10.1038/s41598-021-81213-8. ISSN 2045-2322. PMC 7809475. PMID 33446900.
- ↑ Choi, Kookhwan; Chun, Jaeyoung; Han, Kyungdo; Park, Seona; Soh, Hosim; Kim, Jihye; Lee, Jooyoung; Lee, Hyun Jung; Im, Jong Pil; Kim, Joo Sung (2019-05-10). "Risk of Anxiety and Depression in Patients with Inflammatory Bowel Disease: A Nationwide, Population-Based Study". Journal of Clinical Medicine. 8 (5): 654. doi:10.3390/jcm8050654. ISSN 2077-0383. PMC 6572298. PMID 31083476.
- 1 2 3 Mikocka-Walus, Antonina; Knowles, Simon R.; Keefer, Laurie; Graff, Lesley (March 2016). "Controversies Revisited: A Systematic Review of the Comorbidity of Depression and Anxiety with Inflammatory Bowel Diseases". Inflammatory Bowel Diseases. 22 (3): 752–762. doi:10.1097/MIB.0000000000000620. ISSN 1536-4844. PMID 26841224. S2CID 6315815. Archived from the original on 2022-01-23. Retrieved 2022-04-12.
External links
- Inflammatory bowel disease at Curlie
| Classification |
|
|---|---|
| External resources |