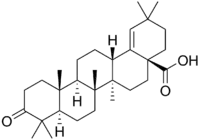
Moronic acid
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(4aS,6aR,6bR,8aR,12aR,12bR,14aS)-2,2,6a,6b,9,9,12a-Heptamethyl-10-oxo-3,4,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14,14a-octadecahydropicene-4a(2H)-carboxylic acid | |
| Other names
Ambronic acid; 3-Oxoolean-18-en-28-oic acid | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C30H46O3 |
| Molar mass | 454.695 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Moronic acid (3-oxoolean-18-en-28-oic acid) is a natural triterpene.[1][2] Moronic acid can be extracted from Rhus javanica, a sumac plant traditionally believed to hold medicinal applications.[2] The molecule has also been extracted from mistletoe (Phoradendron reichenbachianum).[3]
Bevirimat, a derivative of the related triterpenoid betulinic acid, is under development as an anti-HIV drug; however, moronic acid has shown better antiviral profiles in vitro than bevirimat.[4] A particular moronic acid derivative showed potent anti-HIV activity with EC50 values of 0.0085 μM against NL4-3, 0.021 μM against PI-R (a multiple protease inhibitor resistant strain), and 0.13 μM against FHR-2 (an HIV strain resistant to (bevirimat). This derivative has become a new lead for clinical trials and is also active against herpes simplex virus 1.[4]
References
- ↑ "Comparative Toxicogenomics Database: moronic acid".
- 1 2 Kurokawa, Masahiko; Basnet, Purusotam; Ohsugi, Mizue; Hozumi, Toyoharu; Kadota, Shigetoshi; Namba, Tsuneo; Kawana, Takashi; Shiraki, Kimiyasu (1999). "Anti-Herpes Simplex Virus Activity of Moronic Acid Purified from Rhus javanica In Vitro and In Vivo". The Journal of Pharmacology and Experimental Therapeutics. 289 (1): 72–8. PMID 10086989.
- ↑ Rios, María Yolanda; Salinas, David; Villarreal, María Luisa (2001). "Cytotoxic Activity of Moronic Acid and Identification of the New Triterpene 3,4-seco-Olean-18-ene-3,28-dioic Acid from Phoradendron reichenbachianum". Planta Medica. 67 (5): 443–6. doi:10.1055/s-2001-15823. PMID 11488459.
- 1 2 Yu, Donglei; Sakurai, Yojiro; Chen, Chin-Ho; Chang, Fang-Rong; Huang, Li; Kashiwada, Yoshiki; Lee, Kuo-Hsiung (2006). "Anti-AIDS Agents 69. Moronic Acid and Other Triterpene Derivatives as Novel Potent Anti-HIV Agents". Journal of Medicinal Chemistry. 49 (18): 5462–9. doi:10.1021/jm0601912. PMC 2512972. PMID 16942019.