Nardosinone
 | |
| Names | |
|---|---|
| Preferred IUPAC name
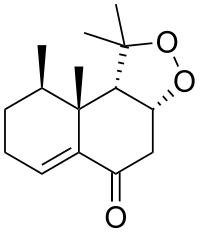
(3aR,9R,9aR,9bS)-1,1,9,9a-Tetramethyl-1,3a,4,7,8,9,9a,9b-octahydro-5H-naphtho[2,1-c][1,2]dioxol-5-one | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C15H22O3 |
| Molar mass | 250.338 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nardosinone is a sesquiterpene and chemical constituent of Nardostachys jatamansi.[1] In in vitro studies, the compound has demonstrated concentration-dependent enhancement of bucladesine and staurosporine-induced neurite outgrowth.[2] Nardosinone has similarly been demonstrated to enhance NGF-mediated neurite outgrowth and synaptogenesis from PC12D cells.[3]
Additionally, nardosinone has demonstrated cytotoxic activity against cultured P-388 lymphocytic leukemia cells.[4]
References
- ↑ Schulte KE, Glauch G, Rücker G (1965). "Nardosinon, ein neuer Inhaltsstoff von Nardostachys chinensis Batalin" [Nardosinone, a new constituent of Nardostachys chinensis Batalin]. Tetrahedron Letters (in German). 6 (35): 3083–3084. doi:10.1016/S0040-4039(01)89226-1. PMID 5828044.
- ↑ Li P, Matsunaga K, Yamakuni T, Ohizumi Y (2003). "Nardosinone, the first enhancer of neurite outgrowth-promoting activity of staurosporine and dibutyryl cyclic AMP in PC12D cells". Developmental Brain Research. 145 (2): 177–183. doi:10.1016/S0165-3806(03)00239-6. PMID 14604758.
- ↑ Li P, Matsunaga K, Yamamoto K, Yoshikawa R, Kawashima K, Ohizumi Y (1999). "Nardosinone, a novel enhancer of nerve growth factor in neurite outgrowth from PC12D cells". Neuroscience Letters. 273 (1): 53–56. doi:10.1016/S0304-3940(99)00629-1. PMID 10505650. S2CID 19913929.
- ↑ Itokawa H, Masuyama K, Morita H, Takeya K (1993). "Cytotoxic sesquiterpenes from Nardostachys chinensis". Chemical & Pharmaceutical Bulletin. 41 (6): 1183–1184. doi:10.1248/cpb.41.1183. PMID 8370115.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.