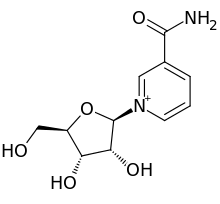
Nicotinamide riboside
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
3-Carbamoyl-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyridin-1-ium | |
| Other names
1-(β-D-Ribofuranosyl)nicotinamide; N-Ribosylnicotinamide | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C11H15N2O5+ |
| Molar mass | 255.25 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nicotinamide riboside (NR, SR647) is a pyridine-nucleoside similar to vitamin B3, functioning as a precursor to nicotinamide adenine dinucleotide or NAD+.[1]
Chemistry
While the molecular weight of nicotinamide riboside is 255.25 g/mol,[2] that of its chloride salt is 290.70 g/mol.[3][4] As such, 100 mg of nicotinamide riboside chloride provides 88 mg of nicotinamide riboside. Recently, the crystal structure of a new series of crystalline forms of other nicotinamide riboside salts has been discovered. These salts correspond to different enantiomers of hydrogen tartrate and hydrogen malate and have a molecular weight of 404.33 and 388.33 g/mol respectively.[5]
History
Nicotinamide riboside (NR) was first described in 1944 as a growth factor, termed Factor V, for Haemophilus influenza, a bacterium that lives in and depends on blood. Factor V, purified from blood, was shown to exist in three forms: NAD+, NMN and NR. NR was the compound that led to the most rapid growth of this bacterium.[6] H. influenza cannot grow on nicotinic acid, nicotinamide, tryptophan or aspartic acid, which were the previously known precursors of NAD+.[7]
In 2000, yeast Sir2 was shown to be an NAD+-dependent protein lysine deacetylase,[8] which led several research groups to probe yeast NAD+ metabolism for genes and enzymes that might regulate lifespan.[9]
In recent years, nicotinamide riboside has been of great interest due to its ability to increase the levels of nicotinamide adenine dinucleotide (NAD+) more than any other NAD+ precursor. During numerous animal and human studies, it was discovered that NR had multiple health benefits, particularly in the treatment several pathophysiological conditions, as well as cardiovascular, neurodegenerative and metabolic disorders. Consequently, NR has been marketed as a nutritional supplement.[10]
Synthesis
Different biosynthetic pathways are responsible for converting the different B3 vitamins into NAD+. The enzyme nicotinamide phosphoribosyltransferase (Nampt) catalyzes the rate-limiting step of the two-step pathway converting nicotinamide to NAD+.[11] NR kinase enzymes can also function as a salvage pathway for NAD+, but this pathway is not essential.[11]
Commercialization
ChromaDex licensed patents in July 2012, and began to develop a process to bring NR to market.[12] ChromaDex has been in a patent dispute with Elysium Health over the rights to nicotinamide riboside supplements.[13]
Biosynth Carbosynth licensed a patent to produce new NR salts WO 2021013795. The new carboxylic acid salts in crystalline form can be used in nutritional supplements and pharmaceutical compositions.
See also
References
- ↑ Bogan, K.L., Brenner, C. (2008). "Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition". Annu. Rev. Nutr. 28: 115–130. doi:10.1146/annurev.nutr.28.061807.155443. PMID 18429699.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ "Nicotinamide riboside". pubchem.ncbi.nlm.nih.gov.
- ↑ "GRAS Notices, GRN No. 635". www.accessdata.fda.gov. Retrieved 18 February 2019.
- ↑ "Spherix/ChromaDex GRAS submission" (PDF). FDA.gov. Retrieved 18 February 2019.
- ↑ Günter, S; et al. (2021). "New Crystalline Salts of Nicotinamide Riboside as Food Additives". Molecules. MDPI. 26 (9): 2729. doi:10.3390/molecules26092729. PMC 8125264. PMID 34066468.
- ↑ Gingrich, W; Schlenk, F (June 1944). "Codehydrogenase I and Other Pyridinium Compounds as V-Factor for Hemophilus influenzae and H. parainfluenzae". Journal of Bacteriology. 47 (6): 535–50. doi:10.1128/JB.47.6.535-550.1944. PMC 373952. PMID 16560803.
- ↑ Belenky, P.; et al. (2007). "NAD+ Metabolism in Health and Disease". Trends in Biochemical Sciences. 32 (1): 12–19. doi:10.1016/j.tibs.2006.11.006. PMID 17161604.
- ↑ Imai, S.; et al. (2000). "Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase". Nature. 403 (6771): 795–800. Bibcode:2000Natur.403..795I. doi:10.1038/35001622. PMID 10693811. S2CID 2967911.
- ↑ Anderson; et al. (2003). "Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae". Nature. 423 (6936): 181–185. Bibcode:2003Natur.423..181A. doi:10.1038/nature01578. PMC 4802858. PMID 12736687.
- ↑ Mehmel, M; Jovanović, N; Spitz, U (2020). "Nicotinamide Riboside — The Current State of Research and Therapeutic Uses". Nutrients. MDPI. 12 (6): 1616. doi:10.3390/nu12061616. PMC 7352172. PMID 32486488.
- 1 2 Fletcher RS, Lavery GG (2018). "The emergence of the nicotinamide riboside kinases in the regulation of NAD+ metabolism". Journal of Molecular Endocrinology. 61 (1): R107–R121. doi:10.1530/JME-18-0085. PMC 6145238. PMID 30307159.
- ↑ "ChromaDex Licenses Exclusive Patent Rights for Nicotinamide Riboside (NR) Vitamin Technologies". 2012-07-16. Retrieved 15 February 2019.
- ↑ Melody M. Bomgardner (2018). "Firms feud over purported age-fighting molecule". Chemical & Engineering News. 96 (33).