Nitroxinil
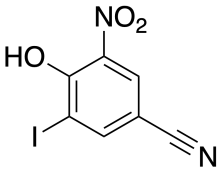 Structure of nitroxinil | |
| Clinical data | |
|---|---|
| Trade names | Fluconix, Dovenix, Trodax |
| Other names | Nitroxynil |
| Routes of administration | Subcutaneous in the form of an N-Ethylglucamine salt solution |
| ATCvet code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.015.350 |
| Chemical and physical data | |
| Formula | C7H3IN2O3 |
| Molar mass | 290.016 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 136–139 °C (277–282 °F) |
SMILES
| |
InChI
| |
Nitroxinil is an anthelmintic, a veterinary medicine against parasitic worms in sheep and cattle. The substance is active against the liver fluke the Fasciola hepatica and to a lesser extent against thread worms in the gastrointestinal tract.[1] Brand names include Fluconix, Dovenix and Trodax. Nitroxynil is also used against strains of the red gum worm (Haemonchus contortus) that have become resistant to benzimidazoles.
Nitroxinil was invented by May & Baker[2] in the mid 1960s as part of a program into investigation of derivatives of p-hydroxybenzonitrile. In addition to Nitroxynil, the herbicides ioxynil (3,5-diiodo) and bromoxynil (3,5-dibromo) were also invented by the same company. Nitroxynil has a nitro group in addition to a single iodine group.
Nitroxynil is almost insoluble in water. It is usually injected subcutaneously into the animals in the form of the water-soluble ethylglucamine salt.[1] It must not be administered to animals that produce milk for human consumption.[3]
References
- 1 2 "NITROXINIL = NITROXYNIL for veterinary use in CATTLE, SHEEP and GOATS against flukes and roundworms". Retrieved 4 April 2018.
- ↑ GB 1104885, May & Baker, "Method for the Treatment of Helminth Infestations", published 18 Dec 1964, issued 6 Mar 1968
- ↑ "Committee for Veterinary Medicinal Products, Nitroxinil, Summary Report" (PDF). The European Agency for the Evaluation of Medical Products. June 1998. p. 5. Retrieved 4 April 2018.