Omaveloxolone
 | |
| Names | |
|---|---|
| Trade names | Skyclarys |
| Other names | RTA 408 |
IUPAC name
| |
| Clinical data | |
| Main uses | Friedreich's ataxia[1] |
| Side effects | Liver problems, headache, nausea, abdominal pain, tiredness, diarrhea, musculoskeletal pain[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | By mouth |
| Typical dose | 150 mg OD[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status | |
| Chemical and physical data | |
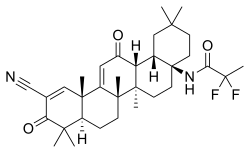
| Formula | C33H44F2N2O3 |
| Molar mass | 554.723 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Omaveloxolone, sold under the brand name Skyclarys, is a medication used to treat Friedreich's ataxia.[1] It is used in those at least 16 years old.[1] It is taken by mouth.[1]
Common side effects include liver problems, headache, nausea, abdominal pain, tiredness, diarrhea, and musculoskeletal pain.[1] Other side effects may include fluid retention or lipid abnormalities.[1] Use in pregnancy may harm the baby.[1] How it works is unclear; however, it appears to activate the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway.[1]
Omaveloxolone was approved for medical use in the United States in 2023.[1] It was granted orphan designation in Europe in 2018.[3] It costs about 370,000 USD per year as of 2023 in the United States.[4]
Medical uses
Omaveloxolone is indicated for the treatment of Friedreich's ataxia.[2][5]
Friedreich's ataxia causes progressive damage to the spinal cord, peripheral nerves, and the brain, resulting in uncoordinated muscle movement, poor balance, difficulty walking, changes in speech and swallowing, and a shortened lifespan.[5] The condition can also cause heart disease.[5] This disease tends to develop in children and teenagers and gradually worsens over time.[5]
Although rare, Friedreich's ataxia is the most common form of hereditary ataxia in the United States, affecting about one in every 50,000 people.[5]
Dosage
It is generally take at a dose of 150 mg once per day.[1]
Mechanism of action
The mechanism of action of omaveloxolone and its related compounds has been demonstrated to be through a combination of activation of the antioxidative transcription factor Nrf2 and inhibition of the pro-inflammatory transcription factor NF-κB.[6]
Nrf2 transcriptionally regulates multiple genes that play both direct and indirect roles in producing antioxidative potential and the production of cellular energy (i.e., adenosine triphosphate or ATP) within the mitochondria. Consequently, unlike exogenously administered antioxidants (e.g., vitamin E or Coenzyme Q10), which provide a specific and finite antioxidative potential, omaveloxolone, through Nrf2, broadly activates intracellular and mitochondrial antioxidative pathways, in addition to pathways that may directly increase mitochondrial biogenesis (such as PGC1α) and bioenergetics.[7]
History
Omaveloxolone is a second generation member of the synthetic oleanane triterpenoid compounds and in clinical development by Reata Pharmaceuticals. Preclinical studies have demonstrated that omaveloxolone possesses antioxidative and anti-inflammatory activities[6][8] and the ability to improve mitochondrial bioenergetics.[7] Omaveloxolone is under clinical investigation for a variety of indications, including Friedreich's ataxia, mitochondrial myopathies, immunooncology, and prevention of corneal endothelial cell loss following cataract surgery.
The efficacy and safety of omaveloxolone was evaluated in a 48-week randomized, placebo-controlled, and double-blind study [Study 1 (NCT02255435)] and an open-label extension.[5] Study 1 enrolled 103 individuals with Friedreich's ataxia who received placebo (52 individuals) or omaveloxolone 150 mg (51 individuals) for 48 weeks.[5] Of the research participants, 53% were male, 97% were white, and the mean age was 24 years at study entry.[5] Nine (18%) patients were younger than age 18.[5] The primary objective was to evaluate the change in the modified Friedreich's Ataxia Rating Scale (mFARS) score compared to placebo at week 48.[5] The mFARS is a clinical assessment that measures disease progression, namely swallowing and speech (bulbar), upper limb coordination, lower limb coordination, and upright stability.[5] Individuals receiving omaveloxolone performed better on the mFARS than people receiving placebo.[5]
The U.S. Food and Drug Administration (FDA) granted the application for omaveloxolone orphan drug, fast track, priority review, and rare pediatric disease designations.[5]
Research
Friedreich's ataxia
There appears to be dose-dependent induction of Nrf2 target genes, along with induction of biomarkers of mitochondrial function in one early clinical trial.[9] Time- and dose-dependent neurological improvements were observed.[9] A phase 1 with patients was completed in 2017 and results from the phase 2 trail where shown in October 2019.[10]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 "DailyMed - SKYCLARYS- omaveloxolone capsule". dailymed.nlm.nih.gov. Archived from the original on 1 July 2023. Retrieved 24 May 2023.
- 1 2 "Archived copy" (PDF). Archived (PDF) from the original on 1 March 2023. Retrieved 1 March 2023.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ "EU/3/18/2037". European Medicines Agency. 17 September 2018. Archived from the original on 12 March 2023. Retrieved 24 May 2023.
- ↑ Buntz, Brian (3 March 2023). "Reata sets $370,000 annual cost for new nerve disorder drug". Drug Discovery and Development. Archived from the original on 7 March 2023. Retrieved 24 May 2023.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 "FDA approves first treatment for Friedreich's ataxia". U.S. Food and Drug Administration (FDA). 28 February 2023. Archived from the original on 1 March 2023. Retrieved 28 February 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 Reisman SA, Lee CY, Meyer CJ, Proksch JW, Ward KW (Jul 2014). "Topical application of the synthetic triterpenoid RTA 408 activates Nrf2 and induces cytoprotective genes in rat skin". Archives of Dermatological Research. 306 (5): 447–54. doi:10.1007/s00403-013-1433-7. PMID 24362512. S2CID 25733020.
- 1 2 Neymotin A, Calingasan NY, Wille E, Naseri N, Petri S, Damiano M, Liby KT, Risingsong R, Sporn M, Beal MF, Kiaei M (Jul 2011). "Neuroprotective effect of Nrf2/ARE activators, CDDO ethylamide and CDDO trifluoroethylamide, in a mouse model of amyotrophic lateral sclerosis". Free Radical Biology & Medicine. 51 (1): 88–96. doi:10.1016/j.freeradbiomed.2011.03.027. PMC 3109235. PMID 21457778.
- ↑ Reisman SA, Lee CY, Meyer CJ, Proksch JW, Sonis ST, Ward KW (May 2014). "Topical application of the synthetic triterpenoid RTA 408 protects mice from radiation-induced dermatitis". Radiation Research. 181 (5): 512–20. Bibcode:2014RadR..181..512R. doi:10.1667/RR13578.1. PMID 24720753. S2CID 23906747.
- 1 2 Strawser, Cassandra; Schadt, Kimberly; Hauser, Lauren; McCormick, Ashley; Wells, McKenzie; Larkindale, Jane; Lin, Hong; Lynch, David R. (26 July 2017). "Pharmacological therapeutics in Friedreich ataxia: the present state". Expert Review of Neurotherapeutics. 17 (9): 895–907. doi:10.1080/14737175.2017.1356721. ISSN 1744-8360. PMID 28724340. S2CID 25424712.
- ↑ "Reata Announces Positive Topline Results from the MOXIe Registrational Trial of Omaveloxolone in Patients with Friedreich's Ataxia" (Press release). 14 October 2019. Archived from the original on 27 November 2022. Retrieved 1 March 2023.
External links
| Identifiers: |
|---|
- Clinical trial number NCT02255435 for "RTA 408 Capsules in Patients With Friedreich's Ataxia - MOXIe" at ClinicalTrials.gov