Oxyrrhis marina
| Oxyrrhis marina | |
|---|---|
 | |
| Scientific classification | |
| Domain: | Eukaryota |
| (unranked): | SAR |
| (unranked): | |
| Phylum: | |
| Class: | |
| Order: | Oxyrrhinales |
| Family: | Oxyrrhinaceae |
| Genus: | Oxyrrhis |
| Species: | O. marina |
| Binomial name | |
| Oxyrrhis marina Dujardin, 1841 | |
Oxyrrhis marina is a species of dinoflagellates with flagella. A marine heterotroph, it is found in much of the world.
Description
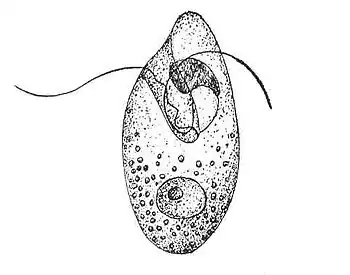
This protozoan species has an asymmetrical oval shape to its single-celled body.[1] It has been likened to a rugby ball.[2] The cell usually measures between 20 and 30 micrometers, but it is known to reach 60. It has two flagella with a protruding, tentacle-like bulge between them. The flagella are covered in scales. Most individuals have scales on the body surface, as well. The two flagella have separate functions. One undulates in waves and the other is coiled, producing a corkscrew-like propulsion to move the cell. The individual appears colorless, but a concentrated culture of cells may have a pink tinge.[1]
Distribution and habitat
The species is thought to have a global distribution except for the polar seas, where it is likely absent or rare, though few samples have been taken of these waters.[3] There are specific records from waters near Europe, North America, Asia, New Zealand, the Canary Islands,[4] Hawaii, and the Azores. It has been found in isolated inland waters, as well, such as a lake in Ukraine. It is less common in the open waters of the oceans. There is a question as to how it came to inhabit so many islands if it is apparently rare in the open ocean. It may have been slowly dispersed on the currents, carried in mats of algae, or transported by humans when shipping arose.[3]
It is most common in the intertidal zone and other coastal regions,[3] where it is a member of the plankton.[5] Habitat types include tide pools and estuaries.[6] It was first described from a salt marsh.[3] It tolerates wide ranges in salinity, temperature, and pH.[7]
Biology
It is heterotrophic, obtaining nutrients externally instead of synthesizing them by an internal process such as photosynthesis. It is an omnivorous grazer, consuming various types of tiny organisms from its environment. It eats phytoplankton such as minute algaes.[8] It has been observed eating Nannochloris oculata and Micromonas pusilla, other flagellates such as Goniomonas amphinema, Pfiesteria piscicida, and Stoeckeria algicida, and some bacteria.[9] It often eats the coccolithophore Cricosphaera elongata, and, in experimental situations, readily eats Tetraselmis suecica, Isochrysis galbana, and Rhodomonas sp. Some of these food items are relatively large, as large as the O. marina cell itself. It is selective in its grazing, showing clear preferences for certain food taxa.[8] It can also pick certain individuals over others, as evidenced by its preference for virus-infected Emiliana huxleyi cells over healthy cells.[10] It is cannibalistic, as well. It feeds by phagocytosis, totally engulfing its prey. It has been observed spinning one of its flagella in such a way that it creates a current, pulling the item closer so it can seize it. It is also raptorial, approaching and pouncing on the prey item, especially when the item is a protist.[9] O. marina can sense and respond to certain chemicals that are exuded by algal prey.[10]
The locomotion of the O. marina cell is helical due to the simultaneous movement of its two flagella. It mostly swims in a straight line, but it makes turns when it detects food.[11]
In terms of reproduction, O. marina is isogamous, with reproductive cells smaller than the body cell, but very little is known about these.[5]
This species sometimes forms red tides,[3][5] but will also feed on the raphidophyte, Heterosigma akashiwo, another organism responsible for red tides.[12] Its blooms when forming red tides are likely stimulated by environmental factors, such as drops in salinity or increases in prey abundance.[5] O. marina may also affect the environment by producing dimethyl sulfide, which is released when it grazes on some prey types, such as E. huxleyi.[10]
Predators of O. marina include protozoa such as the ciliate Strombidinopsis jeokjo, copepods such as Acartia tonsa[10] and rotifers. The mixotrophic flagellate Prymnesium parvum is a prey item for O. marina when the former is nutrient-replete, but can become a predator when it is nutrient-stressed[13] It has been used as food for fish larvae, including those of black porgy (Mylio macrocephalus), lemonpeel angelfish (Centropyge flavissima), and grey mullet (Mugil cephalus). Bryozoans have been grown on a mixture of the protist and yeast.[13]
Research
This protist has been studied extensively. It is a model organism for the study of many aspects of protist biology, including feeding behavior,[8][9] physiology,[1] ecology,[5][7] growth,[5] trophic position,[7] evolution, genomics, and biogeography.[6] Many more studies of its genetics are now underway.[7] There are some limitations to using the species as a model, in part because dinoflagellates are so diverse. O. marina itself is very diverse, with many varied strains, and their biology is influenced by the environment, so it can be hard to find a representative specimen to use as a model.[14] In fact, some experts deny that it is a dinoflagellate at all, or at least a "true" dinoflagellate.[15] In general, it is still very useful for scientific experiments, and researchers recommend it.[14]
O. marina has genes that have evidently been transferred to it from bacteria. It also has some genes that are related to plastids, indicating that it may have had an ancestor that could perform photosynthesis. Also, it has some genes related to essential amino acid synthesis, something that is uncommon in heterotrophs, as they usually obtain essential amino acids by eating them.[7]
It is easy to isolate from the environment and easy to grow in the laboratory. Cultures are fed Dunaliella primolecta or any of a number of other readily available protists. Dead E. coli cells can also be used for food. It can also be sustained on a nutritional medium.[2] Cultures can be maintained for years.[6]
Taxonomy
This protist has been called a morphospecies. As it is now understood, it is composed of a number of isolates, some of which are quite distinct.[1][3] There are 50 to 80 wild isolates.[2] In the future some of these could be divided into separate taxa, perhaps on the species level.[3] One of these may become Oxyrrhis maritima. Another called O. tenticulifera may be valid, as well.[1]
References
- 1 2 3 4 5 Lowe, C. D., et al. (2011). Who is Oxyrrhis marina? Morphological and phylogenetic studies on an unusual dinoflagellate. Journal of Plankton Research 33(4) 555-67.
- 1 2 3 Lowe, C. D., et al. (2011). Collection, isolation and culturing strategies for Oxyrrhis marina. Journal of Plankton Research 33(4) 569-78.
- 1 2 3 4 5 6 7 Watts, P. C., et al. (2011). The distribution of Oxyrrhis marina: a global disperser or poorly characterized endemic? Journal of Plankton Research 33(4) 579-89.
- ↑ Guiry, M .D. & G. M. Guiry. 2013. Oxyrrhis marina. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. Accessed 10 June 2013.
- 1 2 3 4 5 6 Montagnes, D. J. S., et al. (2011). Oxyrrhis marina growth, sex and reproduction. Journal of Plankton Research 33(4) 615-27.
- 1 2 3 Montagnes, D. J. S., et al. (2011). An introduction to the special issue: Oxyrrhis marina, a model organism? Journal of Plankton Research 33(4) 549-54.
- 1 2 3 4 5 Lowe, C. D., et al. (2011). The transcriptome of the novel dinoflagellate Oxyrrhis marina (Alveolata: Dinophyceae): response to salinity examined by 454 sequencing. BMC Genomics 12:519.
- 1 2 3 Hansen, F. C., et al. (1996). Grazing in the heterotrophic dinoflagellate Oxyrrhis marina: size selectivity and preference for calcified Emiliania huxleyi cells. Aquatic Microbial Ecology 10 307-13.
- 1 2 3 Roberts, E. C., et al. (2011). Feeding in the dinoflagellate Oxyrrhis marina: linking behaviour with mechanisms. Journal of Plankton Research 33(4) 603-14.
- 1 2 3 4 Breckels, M. N., et al. (2011). The role of dissolved infochemicals in mediating predator–prey interactions in the heterotrophic dinoflagellate Oxyrrhis marina. Journal of Plankton Research 33(4) 629-39.
- ↑ Boakes, D. E., et al. (2011). Analysis and modelling of swimming behaviour in Oxyrrhis marina. Journal of Plankton Research 33(4) 641-49.
- ↑ Jeong, H. J., et al. (2003). Feeding by the heterotrophic dinoflagellate Oxyrrhis marina on the red-tide raphidophyte Heterosigma akashiwo: a potential biological method to control red tides using mass-cultured grazers. The Journal of Eukaryotic Microbiology 50(4) 274-82.
- 1 2 Yang, Z., et al. (2011). The role of Oxyrrhis marina as a model prey: current work and future directions. Journal of Plankton Research 33(4) 665-75.
- 1 2 Davidson, K., et al. (2011). Oxyrrhis marina-based models as a tool to interpret protozoan population dynamics. Journal of Plankton Research 33(4) 651-63.
- ↑ Saldarriaga, J. F., et al. (2003). Multiple protein phylogenies show that Oxyrrhis marina and Perkinsus marinus are early branches of the dinoflagellate lineage. International Journal of Systematic and Evolutionary Microbiology 53(1) 355-65.
Further reading
- Cachon, M., et al. (1988). Ultrastructure of the flagellar apparatus of Oxyrrhis marina. Biology of the Cell 83 159-68.
- Lowe C. D., et al. (2010). High genetic diversity and fine-scale spatial structure in the marine flagellate Oxyrrhis marina (Dinophyceae) uncovered by microsatellite loci. PLoS ONE 5(12) e15557.
- Opik, H. and K. J. Flynn. (1989). The digestive process of the dinoflagellate, Oxyrrhis marina Dujardin, feeding on the chlorophyte, Dunaliella primolecta Butcher: A combined study of ultrastructure and free amino acids. New Phytologist 113(2) 143-51.
- Single-cell marine predator's unique survival mechanisms revealed: UBC research. Media Release. Public Affairs. University of British Columbia. February 8, 2011.
- Slamovits, C. H. and P. J. Keeling. (2011). Contributions of Oxyrrhis marina to molecular biology, genomics and organelle evolution of dinoflagellates. Journal of Plankton Research 33(4) 591-602.