PCSK9 inhibitors
| PCSK9 inhibitors | |
|---|---|
| Drug class |
PCSK9 inhibitors are a class of medications used to treat hypercholesterolemia.[1][2] In those on high doses of statins, they decrease risk of further problems by 1.5% over 2.5 years.[3]
A number of agents are approved in the United States to treat familial hypercholesterolemia.[4] In Canada they cost about 7,000 CAD per year as of 2018.[3]
Medical uses
PCSK9 inhibitors are promising therapeutics for the treatment of people who exhibit statin intolerance, or as a way to bypass frequent dosage of statins for higher LDL concentration reduction.[5][6]
A review published in 2015 concluded that these agents, when used in patients with high LDL-particle concentrations (thus at greatly elevated risk for cardiovascular disease) seem to be safe and effective at reducing all-cause mortality, cardiovascular mortality, and heart attacks.[7] However a 2020 review concluded that while PCSK9 inhibitor treatment provides additional benefits beyond maximally tolerated statin therapy in high-risk individuals,[8] PCSK9 inhibitor use probably produces little or no difference in mortality.[9]
Prices were very high, inhibiting adoption.[4] The drugs are approved by the FDA for treatment of hypercholesterolemia, notably the genetic condition heterozygous familial hypercholesterolemia which causes high cholesterol levels and heart attacks at a young age.[10] These drugs were later approved by the FDA for the reduction of cardiovascular events including a reduction in all-cause mortality.[11]
Side effects
An FDA warning in March 2014 about possible cognitive adverse effects of PCSK9 inhibition caused concern, as the FDA asked companies to include neurocognitive testing into their Phase III clinical trials.[12]
Types
Monoclonal antibodies
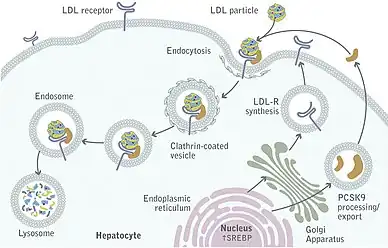
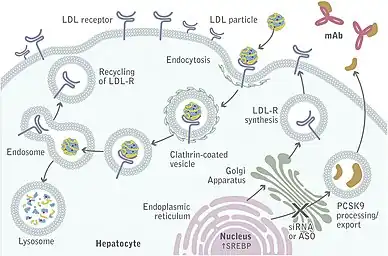
A number of monoclonal antibodies that bind to and inhibit PCSK9 near the catalytic domain were in clinical trials as of 2014. These include evolocumab (Amgen), bococizumab (Pfizer), and alirocumab (Sanofi/Regeneron Pharmaceuticals).[15] As of July 2015, the EU approved these drugs including Evolocumab/Amgen according to Medscape news agency report. A meta-analysis of 24 clinical trials has shown that monoclonal antibodies against PCSK9 can reduce cholesterol, cardiac events and all-cause mortality.[7] The most recent guidelines for cholesterol management from the American Heart Association and American College of Cardiology now provide guidance for when PCSK9 inhibitors should be considered, particularly focusing on cases in which maximally tolerated statin and ezetimibe fail to achieve goal LDL reduction.[16]
A possible side effect of the monoclonal antibody might be irritation at the injection site. Before the infusions, participants received oral corticosteroids, histamine receptor blockers, and acetaminophen to reduce the risk of infusion-related reactions, which by themselves will cause several side effects.[17]
Peptide mimics
Peptides that mimick the EGFA domain of the LDLR that binds to PCSK9 have been developed to inhibit PCSK9.[18]
Gene silencing
Loss-of-function mutations in the PCSK9 gene result in lower levels of LDL and protection against cardiovascular disease.[19] The PCSK9 antisense oligonucleotide increases expression of the LDLR and decreases circulating total cholesterol levels in mice.[20] A locked nucleic acid reduced PCSK9 mRNA levels in mice.[21][22] Initial clinical trials showed positive results of ALN-PCS, which acts by means of RNA interference.[23][24]
In 2021, scientists demonstrated that CRISPR gene editing can decrease blood levels of LDL cholesterol in vivo in Macaca fascicularis monkeys for months by 60 % via knockdown of PCSK9 in the liver.[25][26]
Vaccine
A vaccine that targets PCSK9 has been developed to treat high LDL-particle concentrations. The vaccine uses a VLP (virus-like particle) as an immunogenic carrier of an antigenic PCSK9 peptide. VLP's are viruses that have had their DNA removed so that they retain their external structure for antigen display but are unable to replicate; they can induce an immune response without causing infection. Mice and macaques vaccinated with bacteriophage VLPs displaying PCSK9-derived peptides developed high-titer IgG antibodies that bound to circulating PCSK9. Vaccination was associated with significant reductions in total cholesterol, free cholesterol, phospholipids, and triglycerides.[27]
Naturally occurring
The plant alkaloid berberine inhibits the transcription of the PCSK9 gene in immortalized human hepatocytes in vitro,[28] and lowers serum PCSK9 in mice and hamsters in vivo.[29] It has been speculated[29] that this action contributes to the ability of berberine to lower serum cholesterol.[30] Annexin A2, an endogenous protein, is a natural inhibitor of PCSK9 activity.[31]
References
- ↑ Hlatky MA, Kazi DS (November 2017). "PCSK9 Inhibitors: Economics and Policy". Journal of the American College of Cardiology. 70 (21): 2677–2687. doi:10.1016/j.jacc.2017.10.001. PMID 29169476.
- ↑ Norata GD, Tavori H, Pirillo A, Fazio S, Catapano AL (October 2016). "Biology of proprotein convertase subtilisin kexin 9: beyond low-density lipoprotein cholesterol lowering". Cardiovascular Research. 112 (1): 429–442. doi:10.1093/cvr/cvw194. PMC 5031950. PMID 27496869.
- 1 2 Ton, Joey (3 July 2018). "#215 PCSK9 Inhibitors: Cardiovascular prevention panacea or pesky, pricey pokes?". CFPCLearn. Archived from the original on 28 March 2023. Retrieved 16 June 2023.
- 1 2 Kolata, Gina (2 October 2018). "These Cholesterol-Reducers May Save Lives. So Why Aren't Heart Patients Getting Them?". The New York Times. Archived from the original on 31 March 2023. Retrieved 21 May 2023.
- ↑ Stein EA, Raal FJ (December 2014). "New therapies for reducing low-density lipoprotein cholesterol". Endocrinology and Metabolism Clinics of North America. 43 (4): 1007–1033. doi:10.1016/j.ecl.2014.08.008. PMID 25432394.
- ↑ Vogel RA (June 2012). "PCSK9 inhibition: the next statin?". Journal of the American College of Cardiology. 59 (25): 2354–2355. doi:10.1016/j.jacc.2012.03.011. PMID 22465426.
- 1 2 Navarese EP, Kolodziejczak M, Schulze V, Gurbel PA, Tantry U, Lin Y, et al. (July 2015). "Effects of Proprotein Convertase Subtilisin/Kexin Type 9 Antibodies in Adults With Hypercholesterolemia: A Systematic Review and Meta-analysis". Annals of Internal Medicine. 163 (1): 40–51. doi:10.7326/M14-2957. PMID 25915661. S2CID 207538324.
- ↑ Durairaj A, Sabates A, Nieves J, Moraes B, Baum S (August 2017). "Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) and Its Inhibitors: a Review of Physiology, Biology, and Clinical Data". Current Treatment Options in Cardiovascular Medicine. 19 (8): 58. doi:10.1007/s11936-017-0556-0. PMID 28639183. S2CID 25301414.
- ↑ Schmidt AF, Carter JL, Pearce LS, Wilkins JT, Overington JP, Hingorani AD, Casas JP (October 2020). "PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease". The Cochrane Database of Systematic Reviews. 10 (12): CD011748. doi:10.1002/14651858.CD011748.pub3. PMC 8094613. PMID 33078867.
- ↑ Sijbrands, EJ; Westendorp, RG; Defesche, JC; de Meier, PH; Smelt, AH; Kastelein, JJ (28 April 2001). "Mortality over two centuries in large pedigree with familial hypercholesterolaemia: family tree mortality study". BMJ (Clinical research ed.). 322 (7293): 1019–23. doi:10.1136/bmj.322.7293.1019. PMID 11325764.
- ↑ Wendling P (30 April 2019). "FDA Expands Indication for PCSK9 Alirocumab (Praluent)". Medscape. Archived from the original on 1 February 2023. Retrieved 16 June 2023.
- ↑ Carroll J (7 March 2014). "Regeneron, Sanofi and Amgen shares suffer on FDA's frets about PCSK9 class". FierceBiotech. Archived from the original on 2016-03-04. Retrieved 2023-06-16.
- ↑ Lambert G, Sjouke B, Choque B, Kastelein JJ, Hovingh GK (December 2012). "The PCSK9 decade". Journal of Lipid Research. 53 (12): 2515–2524. doi:10.1194/jlr.R026658. PMC 3494258. PMID 22811413.
- ↑ Lambert G, Sjouke B, Choque B, Kastelein JJ, Hovingh GK (December 2012). "The PCSK9 decade". Journal of Lipid Research. 53 (12): 2515–2524. doi:10.1194/jlr.R026658. PMC 3494258. PMID 22811413.
- ↑ Lambert G, Sjouke B, Choque B, Kastelein JJ, Hovingh GK (December 2012). "The PCSK9 decade". Journal of Lipid Research. 53 (12): 2515–2524. doi:10.1194/jlr.R026658. PMC 3494258. PMID 22811413.
- ↑ Alenghat FJ, Davis AM (February 2019). "Management of Blood Cholesterol". JAMA. 321 (8): 800–801. doi:10.1001/jama.2019.0015. PMC 6679800. PMID 30715135.
- ↑ Fitzgerald K, Frank-Kamenetsky M, Shulga-Morskaya S, Liebow A, Bettencourt BR, Sutherland JE, et al. (January 2014). "Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial". Lancet. 383 (9911): 60–68. doi:10.1016/S0140-6736(13)61914-5. PMC 4387547. PMID 24094767.
- ↑ Shan L, Pang L, Zhang R, Murgolo NJ, Lan H, Hedrick JA (October 2008). "PCSK9 binds to multiple receptors and can be functionally inhibited by an EGF-A peptide". Biochemical and Biophysical Research Communications. 375 (1): 69–73. doi:10.1016/j.bbrc.2008.07.106. PMID 18675252.
- ↑ Cohen JC, Boerwinkle E, Mosley TH, Hobbs HH (March 2006). "Sequence variations in PCSK9, low LDL, and protection against coronary heart disease". The New England Journal of Medicine. 354 (12): 1264–1272. doi:10.1056/NEJMoa054013. PMID 16554528.
- ↑ Graham MJ, Lemonidis KM, Whipple CP, Subramaniam A, Monia BP, Crooke ST, Crooke RM (April 2007). "Antisense inhibition of proprotein convertase subtilisin/kexin type 9 reduces serum LDL in hyperlipidemic mice". Journal of Lipid Research. 48 (4): 763–767. doi:10.1194/jlr.C600025-JLR200. PMID 17242417.
- ↑ Gupta N, Fisker N, Asselin MC, Lindholm M, Rosenbohm C, Ørum H, et al. (May 2010). Deb S (ed.). "A locked nucleic acid antisense oligonucleotide (LNA) silences PCSK9 and enhances LDLR expression in vitro and in vivo". PLOS ONE. 5 (5): e10682. Bibcode:2010PLoSO...510682G. doi:10.1371/journal.pone.0010682. PMC 2871785. PMID 20498851.
- ↑ Lindholm MW, Elmén J, Fisker N, Hansen HF, Persson R, Møller MR, et al. (February 2012). "PCSK9 LNA antisense oligonucleotides induce sustained reduction of LDL cholesterol in nonhuman primates". Molecular Therapy. 20 (2): 376–381. doi:10.1038/mt.2011.260. PMC 3277239. PMID 22108858.
- ↑ Fitzgerald K, White S, Borodovsky A, Bettencourt BR, Strahs A, Clausen V, et al. (January 2017). "A Highly Durable RNAi Therapeutic Inhibitor of PCSK9". The New England Journal of Medicine. 376 (1): 41–51. doi:10.1056/NEJMoa1609243. PMC 5778873. PMID 27959715.
- ↑ Frank-Kamenetsky M, Grefhorst A, Anderson NN, Racie TS, Bramlage B, Akinc A, et al. (August 2008). "Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates". Proceedings of the National Academy of Sciences of the United States of America. 105 (33): 11915–11920. Bibcode:2008PNAS..10511915F. doi:10.1073/pnas.0805434105. PMC 2575310. PMID 18695239.
- ↑ "Scientists Gene-Hacked Monkeys to Fix Their Cholesterol". Futurism. Archived from the original on 13 June 2021. Retrieved 13 June 2021.
- ↑ Musunuru K, Chadwick AC, Mizoguchi T, Garcia SP, DeNizio JE, Reiss CW, et al. (May 2021). "In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates". Nature. 593 (7859): 429–434. Bibcode:2021Natur.593..429M. doi:10.1038/s41586-021-03534-y. PMID 34012082. S2CID 234790939.
- ↑ Crossey E, Amar MJ, Sampson M, Peabody J, Schiller JT, Chackerian B, Remaley AT (October 2015). "A cholesterol-lowering VLP vaccine that targets PCSK9". Vaccine. 33 (43): 5747–5755. doi:10.1016/j.vaccine.2015.09.044. PMC 4609631. PMID 26413878.
- ↑ Li H, Dong B, Park SW, Lee HS, Chen W, Liu J (October 2009). "Hepatocyte nuclear factor 1alpha plays a critical role in PCSK9 gene transcription and regulation by the natural hypocholesterolemic compound berberine". The Journal of Biological Chemistry. 284 (42): 28885–28895. doi:10.1074/jbc.M109.052407. PMC 2781434. PMID 19687008.
- 1 2 Dong B, Li H, Singh AB, Cao A, Liu J (February 2015). "Inhibition of PCSK9 transcription by berberine involves down-regulation of hepatic HNF1α protein expression through the ubiquitin-proteasome degradation pathway". The Journal of Biological Chemistry. 290 (7): 4047–4058. doi:10.1074/jbc.M114.597229. PMC 4326815. PMID 25540198.
- ↑ Dong H, Zhao Y, Zhao L, Lu F (April 2013). "The effects of berberine on blood lipids: a systemic review and meta-analysis of randomized controlled trials". Planta Medica. 79 (6): 437–446. doi:10.1055/s-0032-1328321. PMID 23512497.
- ↑ Seidah NG, Poirier S, Denis M, Parker R, Miao B, Mapelli C, et al. (2012). "Annexin A2 is a natural extrahepatic inhibitor of the PCSK9-induced LDL receptor degradation". PLOS ONE. 7 (7): e41865. Bibcode:2012PLoSO...741865S. doi:10.1371/journal.pone.0041865. PMC 3407131. PMID 22848640.