Papanicolaou stain

Papanicolaou stain (also Papanicolaou's stain and Pap stain) is a multichromatic (multicolored) cytological staining technique developed by George Papanicolaou in 1942.[1][2][3] The Papanicolaou stain is one of the most widely used stains in cytology,[1] where it is used to aid pathologists in making a diagnosis. Although most notable for its use in the detection of cervical cancer in the Pap test or Pap smear, it is also used to stain non-gynecological specimen preparations from a variety of bodily secretions and from small needle biopsies of organs and tissues.[4][5] Papanicolaou published three formulations of this stain in 1942, 1954, and 1960.[2]
Usage
Pap staining is used to differentiate cells in smear preparations (in which samples are spread or smeared onto a glass microscope slide)[6] from various bodily secretions and needle biopsies; the specimens may include gynecological smears (Pap smears), sputum, brushings, washings, urine, cerebrospinal fluid,[4] abdominal fluid, pleural fluid, synovial fluid, seminal fluid,[7] fine needle aspirations, tumor touch samples, or other materials containing loose cells.[8][4][9]
The pap stain is not fully standardized and comes in several formulations, differing in the exact dyes used, their ratios, and timing of the process.[2][1] Pap staining is usually associated with cytopathology in which loose cells are examined, but the stain has also been modified and used on tissue slices.[9]
Pap test
Pap staining is used in the Pap smear (or Pap test) and is a reliable technique in cervical cancer screening in gynecology.[10]
Generalized staining method
The classic form of the Papanicolaou stain involves five stains in three solutions.[2][11][12]
- The first staining solution contains haematoxylin which stains cell nuclei.[10][2][12] Papanicolaou used Harris's hematoxylin in all three formulations of the stain he published.[2]
- The second staining solution (designated OG-6), contains Orange G in 95% ethyl alcohol with a small amount of phosphotungstic acid.[12][2] In the OG-6, the OG signifies Orange G and the '6' denotes the concentration of phosphotungstic acid added; other variants are OG-5 and OG-8).[2]
- The third staining solution is composed of three dyes, Eosin Y, Light Green SF yellowish, and Bismarck brown Y in 95% ethyl alcohol with a small amount of phosphotungstic acid and lithium carbonate.[12][2] This solution, designated EA, followed by a number which denotes the proportion of the dyes, other formulations include EA-36, EA-50, and EA-65.[2]
The counterstains are dissolved in 95% ethyl alcohol which prevents cells from over staining which would obscure nuclear detail and cell outlines especially in the case when cells are overlapping on the slide.[3][2] Phosphotungstic acid is added to adjust the pH of counterstains and helps to optimize the color intensity.[2] The EA counterstain contains Bismarck brown and phosphotungstic acid, which when in combination, cause both to precipitate out of solution, reducing the useful life of the mixture.[2]
Results
The stain should result in cells that are fairly transparent so even thicker specimens with overlapping cells can be interpreted.[2] Cell nuclei should be crisp, blue to black on color[12][13] and the chromatin patterns of the nucleus should be well defined. Cell cytoplasm stains blue-green and keratin stains orange in color.[13][5]
Eosin Y stains the superficial epithelial squamous cells, nucleoli, cilia, and red blood cells.[2] Light Green SF yellowish stains the cytoplasm of other cells, other than superficial squamous cells.[2] Superficial cells are orange to pink, and intermediate and parabasal cells are turquoise green to blue.[12]
Ultrafast Papanicolaou stain
Ultrafast Papanicolaou stain is an alternative for the fine needle aspiration samples, developed to achieve comparable visual clarity in significantly shorter time. The process differs in rehydration of the air-dried smear with saline, use 4% formaldehyde in 65% ethanol fixative, and use of Richard-Allan Hematoxylin-2 and Cyto-Stain, resulting in a 90-second process yielding transparent polychromatic stains.[14]
Examples of Papanicolaou stain
.jpg.webp)
.jpg.webp) Squamous Cell Carcinoma, bronchial washing.
Squamous Cell Carcinoma, bronchial washing..jpg.webp)
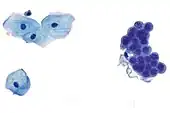 Benign urine cytology sample.
Benign urine cytology sample.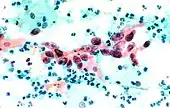 Squamous cell carcinoma in the cervix.
Squamous cell carcinoma in the cervix.
Papers by George N. Papanicolaou describing his stain
- Papanicolaou, George N. "A new procedure for staining vaginal smears." Science 95.2469 (1942): 438–439.
- Papanicolaou, George N. "The cell smear method of diagnosing cancer." American Journal of Public Health and the Nation's Health 38.2 (1948): 202–205.
- Papanicolaou, George N. "Atlas of exfoliative cytology." Published for the commonwealth fund by Harvard University Press. (1954).
- Papanicolaou, George N. "Memorandum on staining." Atlas of exfoliative cytology. Cambridge, MA: Harvard University Press, Supplement II (1960): 12.
See also
References
- 1 2 3 Schulte EK (1991). "Standardization of biological dyes and stains: pitfalls and possibilities". Histochemistry. 95 (4): 319–328. doi:10.1007/BF00266958. PMID 1708749. S2CID 29628388.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Gill, Gary W. (2013). "Papanicolaou Stain". Cytopreparation. Essentials in Cytopathology. Vol. 12. pp. 143–189. doi:10.1007/978-1-4614-4933-1_10. ISBN 978-1-4614-4932-4. ISSN 1574-9053.
- 1 2 Chantziantoniou N, Donnelly AD, Mukherjee M, Boon ME, Austin RM (2017). "Inception and Development of the Papanicolaou Stain Method". Acta Cytol. 61 (4–5): 266–280. doi:10.1159/000457827. PMID 28384641.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - 1 2 3 Kumar, Vinay; Abbas, Abul K.; Aster, Jon C. (2013). Robbins basic pathology (9th ed.). Elsevier/Saunders. p. 910. ISBN 978-1-4377-1781-5.
- 1 2 Drury, R. A. B.; Wallington, E. A. (1980). Carleton's Histological Technique (5th ed.). Oxford University Press. pp. 520p. ISBN 0-19-261310-3.
- ↑ Stedman's medical dictionary (27th ed.). Lippincott Williams & Wilkins. ISBN 978-0683400076.
- ↑ Lars Björndahl; David Mortimer; Christopher L. R. Barratt (1 April 2010). A Practical Guide to Basic Laboratory Andrology. Cambridge University Press. ISBN 978-1-139-48249-3.
- ↑ Hoda RS (2007). "Non-gynecologic cytology on liquid-based preparations: A morphologic review of facts and artifacts". Diagnostic Cytopathology. 35 (10): 621–34. doi:10.1002/dc.20698. PMID 17854077. S2CID 38797168.
- 1 2 Preethi, S.; Sivapathasundharam, B. (2014). "Will modified Papanicolaou stain be the new stain for keratin?". Journal of Histotechnology. 38 (1): 9–13. doi:10.1179/2046023614Y.0000000053. ISSN 0147-8885. S2CID 84486076.
- 1 2 Ross, Michael H.; Pawlina, Wojciech (2016). Histology : a text and atlas : with correlated cell and molecular biology (7th ed.). Wolters Kluwer. pp. 984p. ISBN 978-1451187427.
- ↑ Carson, Freida L; Hladik, Christa (2009). Histotechnology: A Self-Instructional Text (3 ed.). Hong Kong: American Society for Clinical Pathology Press. pp. 361–3363. ISBN 978-0-89189-581-7.
- 1 2 3 4 5 6 Bancroft, John; Stevens, Alan, eds. (1982). The Theory and Practice of Histological Techniques (2nd ed.). Longman Group Limited.
- 1 2 Dey, Pranab (2018). "Routine Staining in Cytology Laboratory". Basic and Advanced Laboratory Techniques in Histopathology and Cytology. pp. 133–138. doi:10.1007/978-981-10-8252-8_14. ISBN 978-981-10-8251-1.
- ↑ Yang GC, Alvarez II (1995). "Ultrafast Papanicolaou stain. An alternative preparation for fine needle aspiration cytology". Acta Cytol. 39 (1): 55–60. PMID 7531380.
- ↑ Demay, Richard (2012). "Chapter 26: Stains". The art and science of cytopathology. Chicago, IL: Am Soc Clinical Pathology. p. 1505. ISBN 978-0-89189-644-9. OCLC 761848930.