Plantazolicin
 | |
| Names | |
|---|---|
| Other names
plantazolicin A, PZN | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
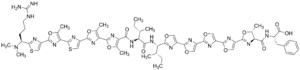
Chemical formula |
C63H69N17O13S2 |
| Molar mass | 1336.47 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Plantazolicin (PZN) is a natural antibiotic produced by the gram-positive soil bacterium Bacillus velezensis FZB42[1] (previously Bacillus amyloliquefaciens FZB42).[2] PZN has specifically been identified as a selective bactericidal agent active against Bacillus anthracis, the causative agent of anthrax. This natural product is a ribosomally synthesized and post-translationally modified peptide (RiPP); it can be classified further as a thiazole/oxazole-modified microcin (TOMM) or a linear azole-containing peptide (LAP).[3]
The significance of PZN stems from its narrow-spectrum antibiotic activity. Most antibiotics in clinical use are broad-spectrum, acting against a wide variety of bacteria, and antibiotic resistance to these drugs is common. In contrast, PZN is antibacterial against only a small number of species, including Bacillus anthracis.
History
The genes for the biosynthesis of PZN were first reported in 2008.[4] The natural product was then isolated in 2011 from Bacillus amyloliquefaciens.[5] The structure of PZN was solved later that year by two independent research groups, primarily through high-resolution mass spectrometry and NMR spectroscopy.[6][7] In 2013, various biomimetic chemical synthesis studies of PZN were reported, including a total synthesis.[8]
Biosynthesis
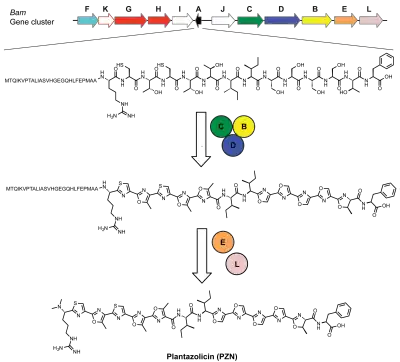
In bacteria, plantazolicin (PZN) is synthesized first as an unmodified peptide via translation at the ribosome. A series of enzymes then chemically alter the peptide to install its post-translational modifications, including several azole heterocycles and an N-terminal amine dimethylation.
Specifically, during the biosynthesis of PZN in B. velezensis, a ribosomally-synthesized precursor peptide undergoes extensive post-translational modification, including cyclodehydrations and dehydrogenations, catalyzed by a trimeric enzyme complex. This process converts cysteine and serine/threonine residues into thiazole and (methyl)oxazole heterocycles[7] (as seen to the right).
The exact mechanism of the association of the trimeric enzyme complex with the N-terminal leader peptide region is not yet understood; however, it is thought that the leader peptide is cleaved from the core peptide putatively by the peptidase contained in the biosynthetic gene cluster.[9] Following leader peptide removal, the newly formed N-terminus undergoes methylation to yield an Nα,Nα-dimethylarginine. This final modification results in mature PZN.
Other organisms such as Bacillus pumilus, Clavibacter michiganensis subsp. sepedonicus, Corynebacterium urealyticum , and Brevibacterium linens have been identified with similar gene clusters that have the potential to produce PZN-like molecules.[7]
References
- ↑ Proteomes - Bacillus velezensis (strain DSM 23117 / BGSC 10A6 / FZB42) (Bacillus amyloliquefaciens subsp. plantarum)
- ↑ Fan, Ben; Wang, Cong; Song, Xiaofeng; Ding, Xiaolei; Wu, Liming; Wu, Huijun; Gao, Xuewen; Borriss, Rainer (2018-10-16). "Bacillus velezensis FZB42 in 2018: The Gram-Positive Model Strain for Plant Growth Promotion and Biocontrol". Frontiers in Microbiology. 9: 2491. doi:10.3389/fmicb.2018.02491. ISSN 1664-302X. PMC 6198173. PMID 30386322.
- ↑ Arnison, Paul G.; Bibb, Mervyn J.; Bierbaum, Gabriele; Bowers, Albert A.; Bugni, Tim S.; Bulaj, Grzegorz; Camarero, Julio A.; Campopiano, Dominic J.; Challis, Gregory L.; Clardy, Jon; Cotter, Paul D.; Craik, David J.; Dawson, Michael; Dittmann, Elke; Donadio, Stefano; Dorrestein, Pieter C.; Entian, Karl-Dieter; Fischbach, Michael A.; Garavelli, John S.; Göransson, Ulf; Gruber, Christian W.; Haft, Daniel H.; Hemscheidt, Thomas K.; Hertweck, Christian; Hill, Colin; Horswill, Alexander R.; Jaspars, Marcel; Kelly, Wendy L.; Klinman, Judith P.; et al. (2013). "Ribosomally synthesized and post-translationally modified peptide natural products: Overview and recommendations for a universal nomenclature". Nat. Prod. Rep. 30 (1): 108–160. doi:10.1039/c2np20085f. PMC 3954855. PMID 23165928.
- ↑ Lee, S. W.; Mitchell, D. A.; Markley, A. L.; Hensler, M. E.; Gonzalez, D.; Wohlrab, A.; Dorrestein, P. C.; Nizet, V.; Dixon, J. E. (2008). "Discovery of a widely distributed toxin biosynthetic gene cluster". Proceedings of the National Academy of Sciences. 105 (15): 5879–5884. doi:10.1073/pnas.0801338105. PMC 2311365. PMID 18375757.
- ↑ Scholz, R.; Molohon, K. J.; Nachtigall, J.; Vater, J.; Markley, A. L.; Sussmuth, R. D.; Mitchell, D. A.; Borriss, R. (2011). "Plantazolicin, a Novel Microcin B17/Streptolysin S-Like Natural Product from Bacillus amyloliquefaciens FZB42". Journal of Bacteriology. 193 (1): 215–224. doi:10.1128/JB.00784-10. PMC 3019963. PMID 20971906.
- ↑ Kalyon, Bahar; Helaly, Soleiman E.; Scholz, Romy; Nachtigall, Jonny; Vater, Joachim; Borriss, Rainer; SüSsmuth, Roderich D. (2011). "Plantazolicin a and B: Structure Elucidation of Ribosomally Synthesized Thiazole/Oxazole Peptides from Bacillus amyloliquefaciensFZB42". Organic Letters. 13 (12): 2996–2999. doi:10.1021/ol200809m. PMID 21568297.
- 1 2 3 Molohon, Katie J.; Melby, Joel O.; Lee, Jaeheon; Evans, Bradley S.; Dunbar, Kyle L.; Bumpus, Stefanie B.; Kelleher, Neil L.; Mitchell, Douglas A. (2011). "Structure Determination and Interception of Biosynthetic Intermediates for the Plantazolicin Class of Highly Discriminating Antibiotics". ACS Chemical Biology. 6 (12): 1307–1313. doi:10.1021/cb200339d. PMC 3241860. PMID 21950656.
- ↑ Banala, Srinivas; Ensle, Paul; Süssmuth, Roderich D. (2013). "Total Synthesis of the Ribosomally Synthesized Linear Azole-Containing Peptide Plantazolicin a from Bacillus amyloliquefaciens". Angewandte Chemie International Edition. 52 (36): 9518–9523. doi:10.1002/anie.201302266. PMID 23761292.
- ↑ Melby, Joel O.; Nard, Nathan J.; Mitchell, Douglas A. (2011). "Thiazole/Oxazole-modified microcins: Complex natural products from ribosomal templates". Current Opinion in Chemical Biology. 15 (3): 369–378. doi:10.1016/j.cbpa.2011.02.027. PMC 3947797. PMID 21429787.