Plinabulin
 | |
| Names | |
|---|---|
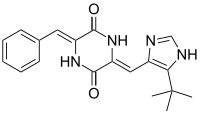
| IUPAC name
(3Z,6Z)-3-Benzylidene-6-{[5-(2-methyl-2-propanyl)-1H-imidazol-4-yl]methylene}-2,5-piperazinedione | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C19H20N4O2 |
| Molar mass | 336.395 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Plinabulin (provisional name BPI-2358, formerly NPI-2358) is a small molecule under development by BeyondSpring Pharmaceuticals, and is in a world-wide Phase 3 clinical trial for non-small cell lung cancer.[1] Plinabulin is being investigated for the reduction of chemotherapy-induced neutropenia[2] and for anti-cancer effects in combination with immune checkpoint inhibitors[3][4] and in KRAS mutated tumors.[5]
Plinabulin blocks the polymerization of tubulin in a unique manner, resulting in multi-factorial effects including an enhanced immune-oncology response,[6] activation of the JNK pathway[7] and disruption of the tumor blood supply.
References
- ↑ "Assessment of Docetaxel + Plinabulin Compared to Docetaxel + Placebo in Patients With Advanced NSCLC With at Least One Measurable Lung Lesion (DUBLIN-3)".
- ↑ Heist, R.S.; Aren, O.R.; Mita, A.C.; Polikoff, J.; Bazhenova, L.; Lloyd, G.K.; Mikrut, W.; Reich, W.; Spear, M.A.; Huang, L. (2014). Randomized Phase 2 Trial of Plinabulin (NPI-2358) Plus Docetaxel in Patients with Advanced Non-Small Lung Cancer (NSCLC). J Clin Oncol 32:5s (abstr 8054).
{{cite conference}}:|format=requires|url=(help) - ↑ "Nivolumab and Plinabulin in Treating Patients With Stage IIIB-IV, Recurrent, or Metastatic Non-small Cell Lung Cancer".
- ↑ Nivolumab in Combination With Plinabulin in Patients With Metastatic Non-Small Cell Lung Cancer (NSCLC).
- ↑ Lloyd, G.K.; Du, L.; Lee, G.; Dalsing-Hernandez, J.; Kotlarczyk, K.; Gonzalez, K.; Nawrocki, S.; Carew, J.; Huang, L. (October 5–9, 2015). Activity of Plinabulin in Tumor Models with Kras Mutations. Proceedings of the International Conference on Molecular Targets and Cancer Therapeutics (Philadelphia (PA) AACR 2015 Abstract nr. 184). Boston MA.
{{cite conference}}:|format=requires|url=(help) - ↑ Lloyd, G.K.; Muller, Ph.; Kashyap, A.; Zippelius, A.; Huang, L. (January 7–9, 2016). Plinabulin: Evidence for an Immune Mediated Mechanism of Action. Proceedings of the Meeting on the Function of Tumor Microenvironment in Cancer Progression (Philadelphia (PA) AACR 2016 Abstract nr A07). San Diego CA.
{{cite conference}}:|format=requires|url=(help) - ↑ Singh, A.V.; Bandi, M.; Raje, N.; Richardson, P.; Palladino, M.A.; Chauhan, D.; Anderson, K. (2011). "A Novel Vascular Disrupting Agent Plinabulin Triggers JNK-Mediated Apoptosis and Inhibits Angiogenesis in Multiple Myeloma Cells". Blood. 117 (21): 5692–5700. doi:10.1182/blood-2010-12-323857. PMC 3110026. PMID 21454451.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.