Pseudohypericin
 | |
| Names | |
|---|---|
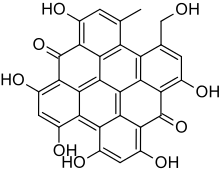
| Preferred IUPAC name
1,3,4,6,8,13-Hexahydroxy-10-(hydroxymethyl)-11-methylphenanthro[3,4,5,6-fghij]perylene-7,14-dione | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.111.993 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C30H16O9 |
| Molar mass | 520.449 g·mol−1 |
| log P | 4.5 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pseudohypericin is an aromatic polycyclic dione that is very closely related to hypericin. It is found most commonly in the St. John's wort family of plants, namely in Hypericum perforatum.[1] In preliminary studies in animal models, pseudohypericin has shown antiviral effects.[2][3] It may also contribute to the potential antidepressant effect of Hypericum perforatum extracts.[4]
References
- ↑ Kitanov, Gerassim M. (2001). "Hypericin and pseudohypericin in some Hypericum species". Biochemical Systematics and Ecology. 29 (2): 171–178. doi:10.1016/S0305-1978(00)00032-6. PMID 11106845.
- ↑ Meruelo, D.; Lavie, G.; Lavie, D. (1988). "Therapeutic agents with dramatic antiretroviral activity and little toxicity at effective doses: Aromatic polycyclic diones hypericin and pseudohypericin". Proceedings of the National Academy of Sciences. 85 (14): 5230–5234. Bibcode:1988PNAS...85.5230M. doi:10.1073/pnas.85.14.5230. PMC 281723. PMID 2839837.
- ↑ Hudson, J.B.; Lopez-Bazzocchi, I.; Towers, G.H.N. (1991). "Antiviral activities of hypericin". Antiviral Research. 15 (2): 101–112. doi:10.1016/0166-3542(91)90028-P. PMID 1650164.
- ↑ Butterweck, Veronika; Petereit, Frank; Winterhoff, Hilke; Nahrstedt, Adolf (1998). "Solubilized Hypericin and Pseudohypericin from Hypericum perforatum Exert Antidepressant Activity in the Forced Swimming Test3". Planta Medica. 64 (4): 291–294. doi:10.1055/s-2006-957437. PMID 9619107.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.