Pulmonary alveolar microlithiasis
| Pulmonary alveolar microlithiasis | |
|---|---|
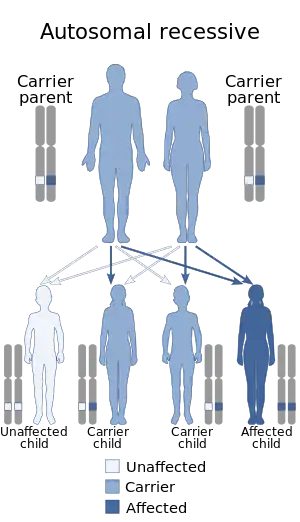 | |
| Pulmonary alveolar microlithiasis is inherited in an autosomal recessive manner | |
| Specialty | Pulmonology |
Pulmonary alveolar microlithiasis (PAM) is a rare, inherited disorder of lung phosphate balance that is associated with small stone formation in the airspaces of the lung. Mutations in the gene SLC34A2[1][2] result in loss of a key sodium, phosphate co-transporter (called Npt2b), known to be expressed in distal alveolar type II cells, as well as in the mammary gland, and to a lesser extent in intestine, kidney, skin, prostate and testes. As the disease progresses, the lung fields become progressively more dense (white) on the chest xray, and low oxygen level, lung inflammation and fibrosis, elevated pressures in the lung blood vessels, and respiratory failure ensue, usually in middle age. The clinical course of PAM can be highly variable, with some patients remaining asymptomatic for decades, and others progressing more rapidly. There is no effective treatment, and the mechanisms of stone formation, inflammation and scarring are not known.
Signs and symptoms
Patients typically have no symptoms until the third or fourth decade of life. In most cases, the disease is discovered incidentally on routine chest Xray. The most common symptoms include the following:[3][4][5][6][7]
- dyspnea
- dry cough
- chest pain
- sporadic hemoptysis
- asthenia
- pneumothoraces
Genetics
PAM is hereditary and another involved family member can be identified in 36% to 61% of cases.[4] Impaired activity of the SLC34A2 gene is responsible for PAM.[1][2][5][8][9][10] The SLC34A2 gene encodes a membrane protein that is expressed primarily in the apical portions of alveolar type II cells[11] and is the most abundant phosphate carrier in the lungs.[5]
Pathogenesis
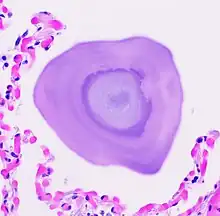
Type II alveolar cells have many important functions in the lung, including the production of pulmonary surfactant, maintenance of fluid balance and composition in the airspace. Phospholipids that make up pulmonary surfactant are broken down by macrophages, releasing phosphate into the alveolar lining fluid. The loss of the Npt2b phosphate transported eliminates the ability of alveolar type II cells to pump phosphorus ions from the alveolar space back into the bloodstream, and leads to microlith formation.[5][12]
Epithelial deletion of Npt2b in mice results in an authentic mimic of the human condition, including accumulation of calcium phosphate microliths in the lung tissue and progressive diffuse radiographic opacities. The mouse model provides a useful platform for preclinical studies, including therapeutic trials of EDTA lavage and low phosphate diet/phosphate binders.[13]
Pathology
PAM may be confined to certain areas or show diffuse distribution through the lungs.[6] Lung biopsy and autopsy specimens demonstrate characteristic intra-alveolar lamellar microliths.[6][14] Calcium deposits in the alveoli begin in the lower lobes and spread over a period of years throughout the lungs.[3]
Diagnosis
PAM is usually diagnosed on the basis of a typical radiological pattern, namely a very fine, sand-like micronodulation of calcific density diffusely involving both lungs, with basal predominance. Many authors argue that this pattern precludes the need for a lung biopsy in most cases.[3][15][16] After PAM is diagnosed in a given patient, family members should be screened by chest radiography, and parents should be counseled that future children are also at risk of developing the disease.[17]
Radiology
Chest radiographs of patients with PAM usually reveal bilateral diffuse micronodular calcifications, producing a "sandstorm” appearance that first involves the inferior portions and then the middle and upper portions of the lungs.[3]
High-resolution computed tomography
The most common findings on HRCT are diffuse hyperdense ground-glass attenuation and subpleural linear calcifications, often most predominant in the inferior and posterior portions of the lungs.[15][18][19][20][21][22] Additionally, the medial aspects of the lungs appear to be more heavily involved than the lateral aspects.[20] Ground-glass opacities, probably due to small calculi in the air space, are the most common finding in children and in patients with early-stage PAM.[19]
Magnetic resonance imaging
On magnetic resonance imaging (MRI), the calcific lesions usually show hypointensity or a signal void on T1- and T2-weighted images.
Pulmonary function studies
Pulmonary function tests, arterial blood gases, ventilation perfusion relationships, and O2 diffusing capacity are normal in the initial stages of PAM. As the disease progresses, pulmonary function tests reveal typical features of a restrictive defect with reduced forced vital capacity (FVC) and elevated forced expiratory volume in FEV1/FVC.
Treatment
To date, no treatment has been proven to effectively reverse or prevent the progression of PAM. Lung transplantation is an option for end stage disease, but is typically only recommended as a last resort when quality of life is significantly impaired.[23]
Etidronate is a bisphosphonate and can reduce the formation of calcium hydroxyapatite crystals. It has led to clinical and radiological improvements in few cases.[24]
Epidemiology
Since the disease was first described in 1918, over 500 case reports have appeared in the literature.[25] PAM is associated with consanguinity. The incidence is higher in Turkey, Japan, India and Italy.[26] The disease affects both men and women equally, and it has been associated with intermarriage within families. [27]The mean age at diagnosis is 35 years based on the cases reported in the literature.
Society
PAM is one of the rare lung diseases currently being studied by the Rare Lung Diseases Consortium (RLDC). Pulmonary Alveolar Microlithiasis patients, families, and caregivers are encouraged to join the NIH Rare Lung Diseases Consortium Contact Registry. This is a privacy protected site that provides up-to-date information for individuals interested in the latest scientific news, trials, and treatments related to rare lung diseases.
References
- 1 2 Huqun; Izumi, S; Miyazawa, H; Ishii, K; Uchiyama, B; Ishida, T; Tanaka, S; Tazawa, R; Fukuyama, S; Tanaka, T; Nagai, Y; Yokote, A; Takahashi, H; Fukushima, T; Kobayashi, K; Chiba, H; Nagata, M; Sakamoto, S; Nakata, K; Takebayashi, Y; Shimizu, Y; Kaneko, K; Shimizu, M; Kanazawa, M; Abe, S; Inoue, Y; Takenoshita, S; Yoshimura, K; Kudo, K; Tachibana, T; Nukiwa, T; Hagiwara, K (2007). "Mutations in the SLC34A2 gene are associated with pulmonary alveolar microlithiasis". Am J Respir Crit Care Med. 175 (3): 263–268. doi:10.1164/rccm.200609-1274oc. PMID 17095743.
- 1 2 Corut, A; Senyigit, A; Ugur, SA; Altin, S; Ozcelik, U; Calisir, H; Yildirim, Z; Gocmen, A; Tolun, A (2006). "Mutations in SLC34A2 cause pulmonary alveolar microlithiasis and are possibly associated with testicular microlithiasis". Am J Hum Genet. 79 (4): 650–656. doi:10.1086/508263. PMC 1592565. PMID 16960801.
- 1 2 3 4 Mariotta, S; Ricci, A; Papale, M; DeClementi, F; Sposato, B; Guidi, L; et al. (2004). "Pulmonary alveolar microlithiasis: report on 576 cases published in the literature". Sarcoidosis Vasc Diffuse Lung Dis. 21 (3): 173–181. PMID 15554073.
- 1 2 Castellana, G; Lamorgese, V (2003). "Pulmonary alveolar microlithiasis. World cases and review of the literature". Respiration. 70 (5): 549–555. doi:10.1159/000074218. PMID 14665786. S2CID 24718986.
- 1 2 3 4 Tachibana, T; Hagiwara, K; Johkoh, T (2009). "Pulmonary alveolar microlithiasis: review and management". Curr Opin Pulm Med. 15 (5): 486–490. doi:10.1097/mcp.0b013e32832d03bb. PMID 19617834. S2CID 39146922.
- 1 2 3 Lauta, VM (2003). "Pulmonary alveolar microlithiasis: an overview of clinical and pathological features together with possible therapies". Respir Med. 97 (10): 1081–1085. doi:10.1016/s0954-6111(03)00140-9. PMID 14561014.
- ↑ Korn, MA; Schurawitzki, H; Klepetko, W; Burghuber, OC (1992). "Pulmonary alveolar microlithiasis: findings on high-resolution CT". AJR Am J Roentgenol. 158 (5): 981–982. doi:10.2214/ajr.158.5.1566701. PMID 1566701.
- ↑ Senyiğit, A; Yaramiş, A; Gürkan, F; Kirbaş, G; Büyükbayram, H; Nazaroğlu, H; Alp, MN; Topçu, F (2001). "Pulmonary alveolar microlithiasis: a rare familial inheritance with report of six cases in a family. Contribution of six new cases to the number of case reports in Turkey". Respiration. 68 (2): 204–209. doi:10.1159/000050494. PMID 11287838. S2CID 72278974.
- ↑ Huqun; Izumi, S; Miyazawa, H; Ishii, K; Uchiyama, B; Ishida, T; et al. (2006). "The autozygous segments predicted by a genome-wide SNP typing revealed mutations in the type IIb sodium phosphate cotransporter (SLC34A2) causing pulmonary alveolar microlithiasis". Proc Am Thorac Soc. 3: A102.
- ↑ Hagiwara, K; Johkoh, T; Tachibana, T (2009). Pulmonary alveolar microlithiasis. New Jersey: Humana Press.
- ↑ Traebert, M; Hattenhauer, O; Murer, H; Kaissling, B; Biber, J (1999). "Expression of type II Na-P(i) cotransporter in alveolar type II cells". Am J Physiol. 277 (5 Pt 1): 868–873. doi:10.1152/ajplung.1999.277.5.L868. PMID 10564169.
- ↑ Poelma, DL; Ju, MR; Bakker, SC; Zimmermann, LJ; Lachmann, BF; van Iwaarden, JF (2004). "A common pathway for the uptake of surfactant lipids by alveolar cells". Am J Respir Cell Mol Biol. 30 (5): 751–758. CiteSeerX 10.1.1.323.3865. doi:10.1165/rcmb.2003-0127oc. PMID 14644915.
- ↑ Saito, A; Nikolaidis, NM; Amlal, H; Uehara, Y; Gardner, JC; LaSance, K; Pitstick, LB; Bridges, JP; Wikenheiser-Brokamp, KA; McGraw, DW; Woods, JC; Sabbagh, Y; Schiavi, SC; Altinişik, G; Jakopović, M; Inoue, Y; McCormack, FX (2015). "Modeling pulmonary alveolar microlithiasis by epithelial deletion of the Npt2b sodium phosphate cotransporter reveals putative biomarkers and strategies for treatment". Sci Transl Med. 7 (313): 313ra181. doi:10.1126/scitranslmed.aac8577. PMC 4764987. PMID 26560359.
- ↑ Barnard, NJ; Crocker, PR; Blainey, AD; Davies, RJ; Ell, SR; Levison, DA (1987). "Pulmonary alveolar microlithiasis. A new analytical approach". Histopathology. 11 (6): 639–645. doi:10.1111/j.1365-2559.1987.tb02674.x. PMID 3623431. S2CID 9063464.
- 1 2 Marchiori, E; Gonçalves, CM; Escuissato, DL; Teixeira, KI; Rodrigues, R; Barreto, MM; Esteves, M (2007). "Pulmonary alveolar microlithiasis: high-resolution computed tomography findings in 10 patients". J Bras Pneumol. 33 (5): 552–557. doi:10.1590/S1806-37132007000500010. PMID 18026653.
- ↑ Sahin, U; Yildiz, M; Bircan, HA; Akkaya, A; Candir, O (2004). "Absence of pulmonary uptake of Tc-99m methylenediphosphonate in alveolar microlithiasis". Ann Nucl Med. 18 (8): 695–698. doi:10.1007/bf02985964. PMID 15682851. S2CID 21539088.
- ↑ Helbich, TH; Wojnarovsky, C; Wunderbaldinger, P; Heinz-Peer, G; Eichler, I; Herold, CJ (1997). "Pulmonary alveolar microlithiasis in children: radiographic and high- resolution CT findings". AJR Am J Roentgenol. 168 (1): 63–65. doi:10.2214/ajr.168.1.8976922. PMID 8976922.
- ↑ Cluzel, P; Grenier, P; Bernadac, P; Laurent, F; Picard, JD (1991). "Pulmonary alveolar microlithiasis: CT findings". J Comput Assist Tomogr. 15 (6): 938–942. doi:10.1097/00004728-199111000-00006. PMID 1939772.
- 1 2 Schmidt, H; Lörcher, U; Kitz, R; Zielen, S; Ahrens, P; König, R (1996). "Pulmonary alveolar microlithiasis in children". Pediatr Radiol. 26 (1): 33–36. doi:10.1007/bf01403701. PMID 8598991. S2CID 21628116.
- 1 2 Deniz, O; Ors, F; Tozkoparan, E; Ozcan, A; Gumus, S; Bozlar, U; Bilgic, H; Ekiz, K; Demirci, N (2005). "High resolution computed tomographic features of pulmonary alveolar microlithiasis". Eur J Radiol. 55 (3): 452–460. doi:10.1016/j.ejrad.2005.01.010. PMID 16129256.
- ↑ Hoshino, H; Koba, H; Inomata, S; Kurokawa, K; Morita, Y; Yoshida, K; Akiba, H; Abe, S (1998). "Pulmonary alveolar microlithiasis: highresolution CT and MR findings". J Comput Assist Tomogr. 22 (2): 245–248. doi:10.1097/00004728-199803000-00016. PMID 9530388.
- ↑ Gasparetto, EL; Tazoniero, P; Escuissato, DL; Marchiori, E; Frare, E; Silva, RL; Sakamoto, D (2004). "Pulmonary alveolar microlithiasis presenting with crazy-paving pattern on high resolution CT". Br J Radiol. 77 (923): 974–976. doi:10.1259/bjr/96331922. PMID 15507428.
- ↑ Stamatis, G; Zerkowski, HR; Doetsch, N; Greschuchna, D; Konietzko, N; Reidemeister, JC (1993). "Sequential bilateral lung transplantation for pulmonary alveolar microlithiasis". Ann Thorac Surg. 56 (4): 972–975. doi:10.1016/0003-4975(93)90370-w. PMID 8215680.
- ↑ Ozcelik, U; Yalcin, E; Ariyurek, M; Ersoz, DD; Cinel, G; Gulhan, B; Kiper, N (2010). "Long-term results of disodium etidronate treatment in pulmonary alveolar microlithiasis". Pediatr Pulmonol. 45 (5): 514–517. doi:10.1002/ppul.21209. PMID 20425862. S2CID 44706545.
- ↑ Mariotta, S; Guidi, L; Mattia, P; Torrelli, L; Pallone, G; Pedicelli, G; Bisetti, A (1997). "Pulmonary microlithiasis. Report of two cases". Respiration. 64 (2): 165–169. doi:10.1159/000196663. PMID 9097354.
- ↑ Castellana, G; Gentile, M; Castellana, R; Fiorente, P; Lamorgese, V (2002). "Pulmonary alveolar microlithiasis: clinical features, evolution of the phenotype, and review of the literature". Am J Med Genet. 111 (2): 220–224. doi:10.1002/ajmg.10530. PMID 12210357.
- ↑ "Rare Lung Diseases > Learn More > Disorder Definitions".