QS-21
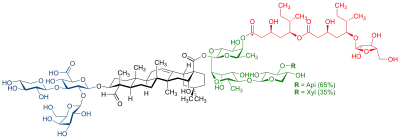
QS-21 is a purified plant extract used as a vaccine adjuvant. It is derived from the soap bark tree (Quillaja saponaria), which is native to the countries of Chile, Peru, and Bolivia.[1] The crude drug (Quillajae cortex) is imported from Peru and Chile.[2]
The extract contains water-soluble triterpene glycosides, which are members of a family of plant-based compounds called saponins. It has been tested as an adjuvant in various vaccines in attempts to improve their efficacy. It is believed to enhance both humoral and cell-mediated immunity.[1]
Isolation of QS-21 destroys the soap bark tree, which has resulted in regulation of the tree by the governments where it is grown. A semi-synthesis strategy relies on purifying the prosapogenin (triterpene and branched trisaccharide) part of the molecule and adding the rest of QS-21 synthetically; this is reported to increase the yield by 2 orders of magnitude.[1] This semi-synthetic approach has also facilitated experimentation with alternative acyl chain compositions.[3]
QS-21 has undergone clinical evaluation as an additive for various trial vaccines, including those for HIV, malaria and cancer. As of 2002, it had been tested in more than 3000 patients in 60 clinical trials. It is a component of the Shingrix vaccine.[4]
QS-21 (Matrix M) is a critical, saponin-derived immunologic adjuvant for the manufacturing of the Novavax COVID-19 vaccine. The addition of QS-21 triterpene-glycosides in form of liposomes is enhancing both humoral and cellular immunogenicity. Agenus Inc. is the sole US-manufacturer of an FDA-approved, patented extract. Supplies are tightly controlled, and the US has invoked the US Defense Production Act to preserve vaccine raw materials for its own companies.[5][6]
See also
- Quillaia
References
- 1 2 3 Ragupathi G, Gardner JR, Livingston PO, Gin DY (2013). "Natural and synthetic saponin adjuvant QS-21 for vaccines against cancer". Expert Rev Vaccines. 10 (4): 463–70. doi:10.1586/erv.11.18. PMC 3658151. PMID 21506644.
- ↑ Max Wichtl, ed. (2004). Herbal Drugs and Phytopharmaceuticals: A Handbook for Practice on a Scientific Basis. Medpharm Publishers. p. 492. ISBN 3-88763-100-5.
- ↑ Chea EK, Fernández-Tejada A, Damani P, Adams MM, Gardner JR, Livingston PO, Ragupathi G, Gin DY (2013). "Synthesis and preclinical evaluation of QS-21 variants leading to simplified vaccine adjuvants and mechanistic probes". J Am Chem Soc. 134 (32): 13448–57. doi:10.1021/ja305121q. PMC 3436428. PMID 22866694.
- ↑ "SHINGRIX package insert" (PDF). Food and Drug Administration. Retrieved 7 April 2019.
- ↑ VISWANATH, P. (April 29, 2021) "COVID-19: Raw material crunch pushes vaccine makers to look at indigenization". moneycontrol.com. Retrieved 30 April 2021.
- ↑ Wang Pengfei (March 2021). "Natural and Synthetic Saponins as Vaccine Adjuvants" Vaccines 9(3):222. PMID: 33807582 PMCID: PMC8001307 DOI: 10.3390/vaccines9030222.