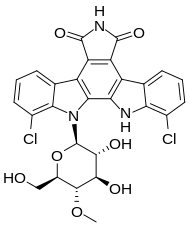
Rebeccamycin
 | |
| Clinical data | |
|---|---|
| Other names | 7,10-dichloro-8-(3,4-dihydroxy-6-(hydroxymethyl)-5-methoxytetrahydro-2H-pyran-2-yl)-8,9-dihydro-1H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-1,3(2H)-dione |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C27H21Cl2N3O7 |
| Molar mass | 570.38 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Rebeccamycin (NSC 655649) is a weak topoisomerase I inhibitor isolated from Nocardia sp. It is structurally similar to staurosporine, but does not show any inhibitory activity against protein kinases. It shows significant antitumor properties in vitro (IC50=480nM against mouse B16 melanoma cells and IC50=500nM against P388 leukemia cells). It is an antineoplastic antibiotic and an intercalating agent.
Becatecarin (BMS-181176) is a synthetic analog of rebeccamycin.[1]
Rebeccamycin and becatecarin have been tested in phase II clinical trials for the treatment of lung cancer, liver cancer, breast cancer, lymphoma, retinoblastoma, kidney cancer, and ovarian cancer.[2]
References
- "Isolation and structure of rebeccamycin - a new antitumor antibiotic from nocardia aerocoligenes": D. E. Nettleton, et al.; Tetrahedron Letters 34, 4011 (1985)
- Production and biological activity of rebeccamycin, a novel antitumor agent: J.A. Bush, et al.; J. Antibiot. (Tokyo) 40, 668 (1987)
- Syntheses and biological activities (topoisomerase inhibition and antitumor and antimicrobial properties) of rebeccamycin analogues bearing modified sugar moieties and substituted on the imide nitrogen with a methyl group F. Anizon, et al.; J. Med. Chem. 40, 3456 (1997)
- DNA cleavage by topoisomerase I in the presence of indolocarbazole derivatives of rebeccamycin: C. Bailly, et al.; Biochemistry 36, 3917 (1997)
- Calories from carbohydrates: energetic contribution of the carbohydrate moiety of rebeccamycin to DNA binding and the effect of its orientation on topoisomerase I inhibition: C. Bailly, et al.; Chem. Biol. 6, 277 (1999)
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.