SR8278
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
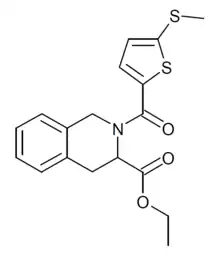
| Formula | C18H19NO3S2 |
| Molar mass | 361.47 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
SR-8278 is an experimental drug that was developed as an antagonist of Rev-ErbAα.[1] It has been used to demonstrate potential applications of Rev-ErbAα antagonists in the treatment of conditions such as Duchenne muscular dystrophy and Alzheimer's disease.[2][3]
See also
References
- ↑ Kojetin D, Wang Y, Kamenecka TM, Burris TP (February 2011). "Identification of SR8278, a synthetic antagonist of the nuclear heme receptor REV-ERB". ACS Chemical Biology. 6 (2): 131–4. doi:10.1021/cb1002575. PMC 3042041. PMID 21043485.
- ↑ Welch RD, Billon C, Valfort AC, Burris TP, Flaveny CA (December 2017). "Pharmacological inhibition of REV-ERB stimulates differentiation, inhibits turnover and reduces fibrosis in dystrophic muscle". Scientific Reports. 7 (1): 17142. Bibcode:2017NatSR...717142W. doi:10.1038/s41598-017-17496-7. PMC 5719458. PMID 29215066.
- ↑ Lee J, Kim DE, Griffin P, Sheehan PW, Kim DH, Musiek ES, Yoon SY (February 2020). "Inhibition of REV-ERBs stimulates microglial amyloid-beta clearance and reduces amyloid plaque deposition in the 5XFAD mouse model of Alzheimer's disease". Aging Cell. 19 (2): e13078. doi:10.1111/acel.13078. PMC 6996949. PMID 31800167.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.