Sivelestat
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | IV |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
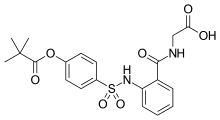
| Formula | C20H22N2O7S |
| Molar mass | 434.46 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Sivelestat (INN, research name ONO 5046, marketed as Elaspol) is an inhibitor of human neutrophil elastase.[1]
It is used in the treatment of acute respiratory failure[2] and preliminary studies show it may also improve neuropathic pain.[3]
Synthesis
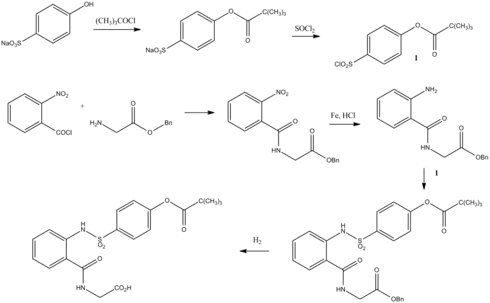
Sivelestat is synthesised as follows:[4]
References
- ↑ Kawabata K, Suzuki M, Sugitani M, Imaki K, Toda M, Miyamoto T (June 1991). "ONO-5046, a novel inhibitor of human neutrophil elastase". Biochem. Biophys. Res. Commun. 177 (2): 814–20. doi:10.1016/0006-291X(91)91862-7. PMID 2049103.
- ↑ Imokawa S, Mori K, Harada M, et al. (June 2008). "[Acute respiratory failure due to pneumocystis pneumonia successfully treated with combined use of sivelestat sodium hydrate]". Nihon Kokyuki Gakkai Zasshi (in Japanese). 46 (6): 461–5. PMID 18592991.
- ↑ Weyer, Andy D.; Stucky, Cheryl L. (May 2015). "Repurposing a leukocyte elastase inhibitor for neuropathic pain". Nature Medicine. 21 (5): 429–430. doi:10.1038/nm.3861. ISSN 1078-8956. PMID 25951529. S2CID 10240018.
- ↑ K. Imaki, Y. Arai, T. Okegawa, U.S. Patent 5,017,610 (1991).
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.