TC-S 7001
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
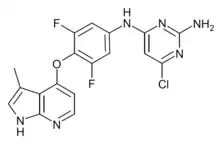
| Formula | C18H13ClF2N6O |
| Molar mass | 402.79 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
TC-S 7001 (Azaindole-1) is a drug which acts as a potent and selective inhibitor of the enzyme Rho kinase, with an IC50 of 0.6 nM at ROCK1 and 1.1 nM at ROCK2.[1] It has vasodilatory effects and has been used in research for a variety of applications.[2][3][4]
See also
References
- ↑ Kast R, Schirok H, Figueroa-Pérez S, Mittendorf J, Gnoth MJ, Apeler H, et al. (December 2007). "Cardiovascular effects of a novel potent and highly selective azaindole-based inhibitor of Rho-kinase". British Journal of Pharmacology. 152 (7): 1070–80. doi:10.1038/sj.bjp.0707484. PMC 2095102. PMID 17934515.
- ↑ Dahal BK, Kosanovic D, Pamarthi PK, Sydykov A, Lai YJ, Kast R, et al. (October 2010). "Therapeutic efficacy of azaindole-1 in experimental pulmonary hypertension". The European Respiratory Journal. 36 (4): 808–18. doi:10.1183/09031936.00140309. PMID 20530035. S2CID 10991200.
- ↑ Pankey EA, Byun RJ, Smith WB, Bhartiya M, Bueno FR, Badejo AM, et al. (July 2012). "The Rho kinase inhibitor azaindole-1 has long-acting vasodilator activity in the pulmonary vascular bed of the intact chest rat". Canadian Journal of Physiology and Pharmacology. 90 (7): 825–35. doi:10.1139/y2012-061. PMID 22591047.
- ↑ Lasker GF, Pankey EA, Allain AV, Murthy SN, Stasch JP, Kadowitz PJ (February 2013). "The selective Rho-kinase inhibitor azaindole-1 has long-lasting erectile activity in the rat". Urology. 81 (2): 465.e7–14. doi:10.1016/j.urology.2012.10.039. PMC 3564057. PMID 23374844.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.