TNP-470
 | |
| Names | |
|---|---|
| Preferred IUPAC name
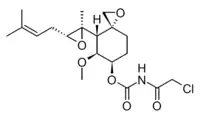
[(3R,4S,5S,6R)-5-methoxy-4-[(2R,3R)-2-methyl-3-(3-methylbut-2-enyl)oxiran-2-yl]-1-oxaspiro[2.5]octan-6-yl] N-(2-chloroacetyl)carbamate | |
| Other names
TNP-470, O-(chloroacetylcarbamoyl)fumagillol | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.189.666 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C19H28ClNO6 |
| Molar mass | 401.88 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
TNP-470 is an methionine aminopeptidase 2 inhibitor. Although it was one of the first angiogenesis inhibitor tested in clinical trials, its potential was hampered by neurotoxic effects and lack of effectiveness.[1][2][3]
References
- ↑ Goya Grocin, Andrea; Kallemeijn, Wouter W.; Tate, Edward W. (October 2021). "Targeting methionine aminopeptidase 2 in cancer, obesity, and autoimmunity". Trends in Pharmacological Sciences. 42 (10): 870–882. doi:10.1016/j.tips.2021.07.004. hdl:10044/1/102175. PMID 34446297. S2CID 237321716.
- ↑ Kruger, E. A.; Figg, W. D. (June 2000). "TNP-470: an angiogenesis inhibitor in clinical development for cancer". Expert Opinion on Investigational Drugs. 9 (6): 1383–1396. doi:10.1517/13543784.9.6.1383. ISSN 1354-3784. PMID 11060750. S2CID 45149960.
- ↑ Stadler, Walter M.; Kuzel, Timothy; Shapiro, Charles; Sosman, Jeffery; Clark, Joseph; Vogelzang, Nicholas J. (August 1999). "Multi-Institutional Study of the Angiogenesis Inhibitor TNP-470 in Metastatic Renal Carcinoma". Journal of Clinical Oncology. 17 (8): 2541–2545. doi:10.1200/JCO.1999.17.8.2541. ISSN 0732-183X. PMID 10561320.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.