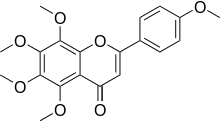
Tangeretin
 | |
 | |
| Names | |
|---|---|
| IUPAC name
4′,5,6,7,8-Pentamethoxyflavone | |
| Preferred IUPAC name
5,6,7,8-Tetramethoxy-2-(4-methoxyphenyl)-4H-1-benzopyran-4-one | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.006.883 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C20H20O7 |
| Molar mass | 372.37 g/mol |
| Density | 1.244 ± 0.06 g/cm3[1] |
| Melting point | 155 to 156 °C (311 to 313 °F; 428 to 429 K) |
| Boiling point | 565.3 ± 50.0 °C (1,049.5 ± 90.0 °F; 838.4 ± 50.0 K)[1] |
Solubility in water |
0.037 g/L[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tangeretin is an O-polymethoxylated flavone that is found in tangerine and other citrus peels. Tangeretin strengthens the cell wall and acts as a plant's defensive mechanism against disease-causing pathogens.[2]
It has also been used as a marker compound to detect contamination in citrus juices.[2]
The following is a list of methods used to extract tangeretin from citrus peels:
- column chromatography
- preparative-high performance liquid chromatography
- super critical fluid chromatography
- high speed counter current chromatography
- a combination of vacuum flash silica gel chromatography and flash C8 column chromatography
- flash chromatography
- isolation using ionic liquids and a cycle of centrifugation and decantation[3]
The low solubility of Tangeretin is one of the main reasons for the low bioavailability of Tangeretin (and other flavonoids in general), and has been reported as a major challenge when using the compound in laboratory procedures.[4] However, methods for tangeretin extraction are currently being tested to maximize efficiency and percent yields as its uses in treatment of cancer and other diseases are becoming better understood.[2]
Tangeretin is commercially available as a dietary supplement. Tangeretin has also demonstrated beneficial applications in other pharmaceutical, nutraceutical, and cosmetic processes.[4]
Tangeretin can be found as various synonyms throughout literature and research, including Tangeritin and 5,6,7,8,4’-pentamethoxyflavone (VIII)[3]
References
- 1 2 3 SciFinder.com (accessed Nov. 6, 2012). Tangeretin (481-53-8).
- 1 2 3 Uckoo, RM; et al. Sep. Purif. Technol. 2011.
- 1 2 Mizuno, H.; Yoshikawa, H.; Usuki, T. Extraction of Nobiletin and Tangeretin From Peels of Shekwasha and Ponkan Using [C2mim][(MeO)(H)PO2] and Centrifugation. Natural Product Communications 2019, 14, 1-6.
- 1 2 Panche, A. N.; Diwan, A. D.; Chandra, S. R. Flavonoids: an overview. Journal of Nutritional Science. 2016, 5, 47.