Taselisib
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
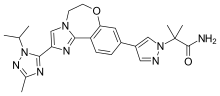
| Formula | C24H28N8O2 |
| Molar mass | 460.542 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Taselisib (development code: GDC-0032) is an experimental cancer drug that was in development by Roche. It is a small molecule inhibitor targeting the p110α protein product of the phosphoinositide 3-kinase gene, PIK3CA.[1][2]
Roche announced in June 2018 that there would be no further development of taselislib following the top line results of the Phase III "Sandpiper" study.[3] Currently running clinical trials[4] were continued for patients exhibiting benefit.
References
- ↑ "Definition of taselisib - NCI Drug Dictionary - National Cancer Institute". Cancer.gov. Retrieved 2017-01-10.
- ↑ Zumsteg ZS, Morse N, Krigsfeld G, Gupta G, Higginson DS, Lee NY, et al. (April 2016). "Taselisib (GDC-0032), a Potent β-Sparing Small Molecule Inhibitor of PI3K, Radiosensitizes Head and Neck Squamous Carcinomas Containing Activating PIK3CA Alterations". Clinical Cancer Research. 22 (8): 2009–19. doi:10.1158/1078-0432.CCR-15-2245. PMC 4870591. PMID 26589432.
- ↑ "Roche dumps its PhIII PI3K effort on taselisib after researchers track poor survival edge, harsh side effects for breast cancer".
- ↑ https://clinicaltrials.gov/ct2/results?term=taselisib&Search=Apply&recrs=d&age_v=&gndr=&type=&rslt=
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.