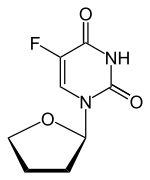
Tegafur/uracil
| |||
| Combination of | |||
|---|---|---|---|
| Tegafur | Antineoplastic agent | ||
| Uracil | Nucleobase | ||
| Clinical data | |||
| Trade names | Uftoral, others | ||
| Identifiers | |||
| CAS Number | |||
| PubChem CID | |||
| DrugBank | |||
| UNII | |||
Tegafur/uracil is a chemotherapy drug combination used in the treatment of cancer, primarily bowel cancer. It is also called UFT or UFUR.
Description
UFT is a first generation dihydropyrimidine dehydrogenase (DPD) inhibitory fluoropyrimidine drug. It is an oral agent which combines uracil, a competitive inhibitor of DPD, with the 5-fluorouracil (5-FU) prodrug tegafur in a 4:1 molar ratio.
Mechanism of action
Excess uracil competes with 5-FU for DPD, thus inhibiting 5-FU catabolism. The tegafur is taken up by the cancer cells and breaks down into 5-FU, a substance that kills tumor cells. The uracil causes higher amounts of 5-FU to stay inside the cells and kill them. Tegafur is a type of antimetabolite. Uracil has also been stated to help protect the gastrointestinal tract from 5-FU toxicity and the related metabolites, with less side effects than 5-FU and other 5-FU related (pro)drugs. Tetrahydrofuran metabolites from the tegafur metabolism, unique among 5-FU based drugs, have also been shown to improve the antiangiogenic and cytocidal performance of 5-FU, particularly in patients with over-expressed HIF-1.
Trial results
Trials using UFT for cancer treatment include pancreatic cancer, colorectal cancer,[1][2] liver cancer,[3] adenocarcinoma of the lung[4] and breast cancer[5][6] with significant gains over existing treatments, with reduced side effects, improved quality of life, improved disease free survival and/or overall survival.
History
The UFT combination was developed in Japan during the 1980s. UFT is approved in over 50 countries as a cancer therapy, most commonly for advanced colorectal cancer to replace 5FU, and has a low cost.[1] "[P]atients appeared strongly to prefer treatment with [oral] UFT/LV over [intravenous] 5-FU/LV."[7] In Japan, UFT is approved for cancer treatments including tumors of the colon/rectum, lung, breast, stomach, head and neck, liver, gallbladder, bile duct, pancreas, bladder, prostate, and cervix.[8] In the UK, tegafur/uracil with folinic acid is approved as first line treatment by the National Institute for Health and Clinical Excellence (NICE) for metastatic colorectal cancer.[9]
Manufacturing and marketing
Tegafur/uracil is marketed by companies including Merck Serono, Korea United and Jeil, Taiho, mostly in Asia, Europe, South America, Central America and South Africa.
It is made by various manufacturers and sold under a variety of names including: Tegafur-uracil, UFT, Ftorafur, Tefudex, Ufur and Uftoral. The UFT brand version is authorized for marketing in over 50 countries. Between 1984 and 2006, over 30 million patients were treated with UFT.[10]
References
- 1 2 Akasu T, Moriya Y, Ohashi Y, Yoshida S, Shirao K, Kodaira S (April 2006). "Adjuvant chemotherapy with uracil-tegafur for pathological stage III rectal cancer after mesorectal excision with selective lateral pelvic lymphadenectomy: a multicenter randomized controlled trial". Jpn. J. Clin. Oncol. 36 (4): 237–44. doi:10.1093/jjco/hyl014. PMID 16675478.
- ↑ Casado E, Pfeiffer P, Feliu J, González-Barón M, Vestermark L, Jensen HA (August 2008). "UFT (tegafur-uracil) in rectal cancer". Ann. Oncol. 19 (8): 1371–8. doi:10.1093/annonc/mdn067. PMID 18381370.
- ↑ Ishikawa T (May 2008). "Chemotherapy with enteric-coated tegafur/uracil for advanced hepatocellular carcinoma". World J Gastroenterol. 14 (18): 2797–2801. doi:10.3748/wjg.14.2797. PMC 2710718. PMID 18473401.
- ↑ Kato H, Ichinose Y, Ohta M, et al. (April 2004). "A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung". N. Engl. J. Med. 350 (17): 1713–21. doi:10.1056/NEJMoa032792. PMID 15102997.
- ↑ Watanabe T, Sano M, Takashima S, et al. (March 2009). "Oral uracil and tegafur compared with classic cyclophosphamide, methotrexate, fluorouracil as postoperative chemotherapy in patients with node-negative, high-risk breast cancer: National Surgical Adjuvant Study for Breast Cancer 01 Trial". J. Clin. Oncol. 27 (9): 1368–74. doi:10.1200/JCO.2008.18.3939. PMID 19204202.
- ↑ Nakayama T, Noguchi S (2010). "Therapeutic usefulness of postoperative adjuvant chemotherapy with Tegafur-Uracil (UFT) in patients with breast cancer: focus on the results of clinical studies in Japan". Oncologist. 15 (1): 26–36. doi:10.1634/theoncologist.2009-0255. PMC 3227888. PMID 20080863.
- ↑ "Archived copy". www.hta.ac.uk. Archived from the original on 18 November 2009. Retrieved 15 January 2022.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ "Merck at ASCO 2007: New Data Demonstrate the Value of UFT as an Important Treatment Option for Patients With Metastatic Colorectal Cancer - Drugs.com MedNews". Drugs.com.
- ↑ Capecitabine and tegafur uracil for metastatic colorectal cancer, Technical appraisal 61
- ↑ Merck at ASCO 2007: New Data Demonstrate the Value of UFT as an Important Treatment Option for Patients With Metastatic Colorectal Cancer, June 2007, Merck's press-release
External links
- Murad A, de Andrade CA, Delfino C, Arikian S, Doyle J, Sinha N (September 1997). "A pharmacoeconomic comparison of UFT and 5-FU chemotherapy for colorectal cancer in South America". Oncology (Williston Park, N.Y.). 11 (9 Suppl 10): 128–35. PMID 9348585.
- Yoshitani S, Takashima S (February 2009). "Efficacy of postoperative UFT (Tegafur/Uracil) plus PSK therapies in elderly patients with resected colorectal cancer". Cancer Biother. Radiopharm. 24 (1): 35–40. doi:10.1089/cbr.2008.0547. PMID 19243246.
- Sakai T, Yamashita Y, Maekawa T, Mikami K, Hoshino S, Shirakusa T (August 2008). "Immunochemotherapy with PSK and fluoropyrimidines improves long-term prognosis for curatively resected colorectal cancer". Cancer Biother. Radiopharm. 23 (4): 461–7. doi:10.1089/cbr.2008.0484. PMID 18771350.
- Sadahiro S, Mitomi T, Noto T, et al. (July 2005). "[Multicenter comparative study of the recurrence-inhibitory effect of oral fluoropyrimidine drugs in patients with colorectal cancer following curative resection]". Gan to Kagaku Ryoho (in Japanese). 32 (7): 997–1005. PMID 16044962.
- Shirao K, Hoff PM, Ohtsu A, et al. (September 2004). "Comparison of the efficacy, toxicity, and pharmacokinetics of a uracil/tegafur (UFT) plus oral leucovorin (LV) regimen between Japanese and American patients with advanced colorectal cancer: joint United States and Japan study of UFT/LV". J. Clin. Oncol. 22 (17): 3466–74. doi:10.1200/JCO.2004.05.017. PMID 15277535.
- Onoyama H, Urakawa T, Sugihara S, et al. (October 2000). "[A noteworthy case of postoperative liver metastasis from gastric cancer which responded well to UFT therapy]". Gan to Kagaku Ryoho (in Japanese). 27 (11): 1731–5. PMID 11057325.
- Matsushita A, Hanazaki K, Noike T, et al. (September 2003). "[Complete disappearance with oral UFT administration of recurrent hepatocellular carcinoma of the remnant liver and multiple lung metastasis after hepatic resection]". Gan to Kagaku Ryoho (in Japanese). 30 (9): 1327–32. PMID 14518415.
- Ohashi Y, Watanabe M, Ikeda M, et al. (December 2008). "[Regression of metastatic hepatic cancer from gastric cancer by polysaccharide K and UFT administration—a case report]". Gan to Kagaku Ryoho (in Japanese). 35 (13): 2409–12. PMID 19098413.
- Nakagawa Y, Todoroki T, Morishita Y, et al. (2008). "A long-term survivor after pancreaticoduodenectomy for metastatic undifferentiated carcinoma of an unknown primary". Hepatogastroenterology. 55 (86–87): 1557–61. PMID 19102342.
- Ueda H, Tanaka H, Kida Y, Fukuchi H, Ichinose M (May 2008). "Adjuvant chemotherapy with tegafur/uracil administration after transcatheter arterial chemoembolization for advanced hepatocellular carcinoma". Oncol. Rep. 19 (5): 1355–61. doi:10.3892/or.19.5.1355. PMID 18425398.
- Lin PC, Chen WS, Chao TC, Yang SH, Tiu CM, Liu JH (August 2007). "Biweekly oxaliplatin plus 1-day infusional fluorouracil/leucovorin followed by metronomic chemotherapy with tegafur/uracil in pretreated metastatic colorectal cancer". Cancer Chemother. Pharmacol. 60 (3): 351–6. doi:10.1007/s00280-006-0377-4. PMID 17111120. S2CID 29375226.
- Ohwada S, Ikeya T, Yokomori T, et al. (March 2004). "Adjuvant immunochemotherapy with oral Tegafur/Uracil plus PSK in patients with stage II or III colorectal cancer: a randomised controlled study". Br. J. Cancer. 90 (5): 1003–10. doi:10.1038/sj.bjc.6601619. PMC 2409633. PMID 14997197.