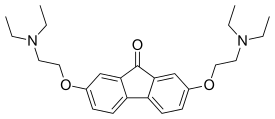
Tilorone
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | 60% |
| Protein binding | ~80% |
| Metabolism | Nil |
| Elimination half-life | 48 hours |
| Excretion | Feces (70%), urine (9%)[1] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C25H34N2O3 |
| Molar mass | 410.558 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Tilorone (trade names Amixin, Lavomax and others) is the first recognized synthetic, small molecular weight compound that is an orally active interferon inducer.[2] It is used as an antiviral drug. It is effective against Ebola virus in mice.[3]
Pharmacology
Tilorone activates the production of interferon.[2]
References
- ↑ "Registry of Medicinal Products (RLS). Tilorone: Prescribing Information" (in Russian). Retrieved 2 October 2016.
- 1 2 Stringfellow D, Glasgow L (1972). "Tilorone hydrochloride: an oral interferon-inducing agent". Antimicrob Agents Chemother. 2 (2): 73–8. doi:10.1128/aac.2.2.73. PMC 444270. PMID 4670490.
- ↑ Ekins, S.; Lingerfelt, M. A.; Comer, J. E.; Freiberg, A. N.; Mirsalis, J. C.; O'Loughlin, K.; Harutyunyan, A.; McFarlane, C.; Green, C. E.; Madrid, P. B. (2018). "Efficacy of Tilorone Dihydrochloride against Ebola Virus Infection". Antimicrobial Agents and Chemotherapy. 62 (2). doi:10.1128/AAC.01711-17. PMC 5786809. PMID 29133569.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.