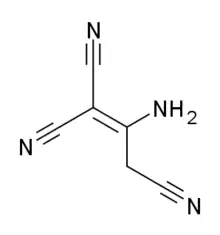
Tricyanoaminopropene
 | |
| Clinical data | |
|---|---|
| Trade names | Triap |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.011.616 |
| Chemical and physical data | |
| Formula | C6H4N4 |
| Molar mass | 132.126 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Tricyanoaminopropene (TRIAP, TCAP, Malononitrile Dimer, 1,1,3-tricyano-2-amino-1-propene) is a nootropic drug which mimics the function of nerve growth factor and increases the growth of nerves and tissue regeneration both in isolated tissues[1] and in vivo. It stimulates the action of the enzyme choline acetyltransferase, resulting in increased acetylcholine production.[2] This then results in increased synthesis of RNA in many different tissues in the body.[3] However it also suppresses the production of thyroxine, causing temporary hypothyroidism which returns to normal once the drug is discontinued.[4]
Tricyanoaminopropene reduces the amnesia produced by electroconvulsive shock,[5] and animal tests suggested nootropic activity,[6][7][8] but no beneficial effect was found when it was tested in mentally retarded children,[9] and administration to pregnant rats actually reduced learning ability in their young because of its anti-thyroid hormone effects.[10] This drug thus produces two effects which oppose each other, with the nootropic effect from the increased acetylcholine production cancelled out by the opposite effect produced by the decrease in production of thyroxine. It is not known whether the efficacy of the drug could be improved by supplementation with additional thyroxine or equivalent drugs.
See also
References
- ↑ Paul JW, Quach TT, Duchemin AM, Schrier BK, DaVanzo JP (August 1990). "1,1,3 tricyano-2-amino-1-propene (Triap): a small molecule which mimics or potentiates nerve growth factor". Brain Research. Developmental Brain Research. 55 (1): 21–7. doi:10.1016/0165-3806(90)90101-4. PMID 2208638.
- ↑ Paul JW, DaVanzo JP (June 1992). "1,1,3 Tricyano-2-amino-1-propene (Triap) stimulates choline acetyltransferase activity in vitro and in vivo". Brain Research. Developmental Brain Research. 67 (2): 113–20. doi:10.1016/0165-3806(92)90212-f. PMID 1511511.
- ↑ Dhindsa KS, Enesco HE (1978). "Radioautographic study of the effect of malononitrile dimer on RNA synthesis in mice". Acta Anatomica. 100 (1): 44–50. doi:10.1159/000144880. PMID 899675.
- ↑ Dhindsa KS (1980). "Histological changes in the thyroid gland of the mouse following treatment with 1, 1, 3-tricyano-2-amino-1-propene". Acta Anatomica. 106 (4): 468–72. doi:10.1159/000145216. PMID 7386168.
- ↑ Essman WB. Effect of tricyanoaminopropene on the amnesic effect of electroconvulsive shock. Psychopharmacology (Berlin. 1966 Jan;9(5):426-433 doi:10.1007/BF00406452.
- ↑ Rahwan RG (November 1971). "The biochemical and pharmacological basis of learning and memory". Agents and Actions. 2 (3): 87–102. doi:10.1007/bf01990087. PMID 4950562. S2CID 38569933.
- ↑ Banfi S, Cornelli U, Fonio W, Dorigotti L (December 1982). "A screening method for substances potentially active on learning and memory". Journal of Pharmacological Methods. 8 (4): 255–63. doi:10.1016/0160-5402(82)90042-0. PMID 7154677.
- ↑ Corson JA. "Studies of the Effects of Manipulation of Brain Metabolism on Learning. I. Vitamin B12. II. Magnesium Pemoline (Cylert). III. Malononitrile Dimer (u9189). IV. Anodal Polarization".
- ↑ Panzer JD, Atkinson WH (1969). "Tricyanoaminopropene (TCAP): lace of improvement of mentation in mentally retarded children". Psychosomatics. 10 (2): 136–40. doi:10.1016/S0033-3182(69)71773-X. PMID 4181970.
- ↑ Davenport JW (February 1970). "Cretinism in rats: enduring behavioral deficit induced by tricyanoaminopropene". Science. New York, N.Y. 167 (3920): 1007–8. Bibcode:1970Sci...167.1007D. doi:10.1126/science.167.3920.1007. PMID 4188980. S2CID 20247454.