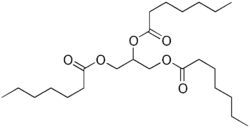
Triheptanoin
 | |
 | |
| Names | |
|---|---|
| Trade names | Dojolvi |
| Other names | UX007 |
IUPAC name
| |
| Clinical data | |
| Drug class | Glycerolipids |
| Main uses | Long-chain fatty acid oxidation disorders (LC-FAOD)[1] |
| Side effects | Abdominal pain, diarrhea, vomiting, nausea[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | By mouth |
| Typical dose | Up to 35% of caloric intake[1] |
| External links | |
| AHFS/Drugs.com | Professional Drug Facts |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
| Formula | C24H44O6 |
| Molar mass | 428.610 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Triheptanoin, sold under the brand name Dojolvi, is a medication used to treat long-chain fatty acid oxidation disorders (LC-FAOD).[1] This includes pyruvate carboxylase deficiency and carnitine palmitoyltransferase II deficiency.[3][4] It is taken by mouth.[1]
Common side effects include abdominal pain, diarrhea, vomiting, and nausea.[1] In those with pancreatic insufficiency intestinal malabsorption may occur.[1] It is a manufactured medium-chain triglyceride and is a source of calories and fatty acids.[1][5]
Triheptanoin was approved for medical use in the United States in 2020.[1] As of 2022 it is not approved in Europe or the United Kingdom.[5] In the United States it costs about 5,600 USD for 500 ml as of 2022.[6]
Medical uses
Dojolvi is indicated as a source of calories and fatty acids for the treatment of children and adults with molecularly confirmed long-chain fatty acid oxidation disorders (LC-FAOD).[1][7]
Dosage
It is given 4 times per day with either food or via a feeding tube.[1] It may be used as up to 35% of a persons caloric intake.[1]
Mechanism of action
Triheptanoin is a triglyceride that is composed of three seven-carbon (C7:0) fatty acids. These odd-carbon fatty acids are able to provide anaplerotic substrates for the TCA cycle. Since triheptanoin is composed of odd-carbon fatty acids, it can produce ketone bodies with five carbon atoms, as opposed to even-carbon fatty acids which are metabolized to ketone bodies with four carbon atoms. The five-carbon ketones produced from triheptanoin are beta-ketopentanoate and beta-hydroxypentanoate. Each of these ketone bodies easily crosses the blood–brain barrier and enters the brain.
History
Triheptanoin was designated an orphan drug by the U.S. Food and Drug Administration (FDA) in 2006, 2008, 2014, and 2015.[8][9][10][11] Triheptanoin was also designated an orphan drug by the European Medicines Agency (EMA).[12][13][14][15][16][17][18][19]
Triheptanoin was approved for medical use in the United States in June 2020.[20][7]
The FDA approved triheptanoin based on evidence from three clinical trials (Trial 1/NCT018863, Trial 2/NCT022141 and Trial 3/NCT01379625).[21] The trials enrolled children and adults with LC-FAOD.[21] Trials 1 and 2 were conducted at 11 sites in the United States and the United Kingdom, and Trial 3 was conducted at two sites in the United States.[21]
Trial 1 and Trial 2 were used to evaluate the side effects of triheptanoin.[21] Both trials enrolled children and adults diagnosed with LC-FAOD.[21] In Trial 1, participants received triheptanoin for 78 weeks.[21] Trial 2 enrolled participants from other trials who were already treated with triheptanoin (including those from Trial 1) as well as participants who were never treated with triheptanoin before.[21] Trial 2 is still ongoing and is planned to last up to five years.[21]
The benefit of triheptanoin was evaluated in Trial 3 which enrolled children and adults with LC-FAOD.[21] Half of the participants received triheptanoin and half received trioctanoin for four months.[21] Neither the participants nor the investigators knew which treatment was given until the end of the trial.[21] The benefit of triheptanoin in comparison to trioctanoin was assessed by measuring the changes in heart and muscle function.[21]
Names
Triheptanoin is the international nonproprietary name.[22]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 "Dojolvi- triheptanoin liquid". DailyMed. 30 June 2020. Archived from the original on 2 August 2021. Retrieved 24 September 2020.
- ↑ "Summary Basis of Decision (SBD) for Dojolvi". Health Canada. Archived from the original on 30 May 2022. Retrieved 29 May 2022.
- ↑ "Pyruvate Carboxylase Deficiency". NORD (National Organization for Rare Disorders). Archived from the original on 23 October 2022. Retrieved 4 November 2022.
- ↑ RESERVED, INSERM. "Orphanet: Carnitine palmitoyl transferase II deficiency, myopathic form". www.orpha.net. Archived from the original on 27 November 2021. Retrieved 4 November 2022.
- 1 2 "Triheptanoin". SPS - Specialist Pharmacy Service. 2 February 2018. Archived from the original on 31 January 2022. Retrieved 4 November 2022.
- ↑ "Dojolvi Prices, Coupons, Copay & Patient Assistance". Drugs.com. Archived from the original on 16 September 2021. Retrieved 4 November 2022.
- 1 2 "Ultragenyx Announces U.S. FDA Approval of Dojolvi (UX007/triheptanoin), the First FDA-Approved Therapy for the Treatment of Long-chain Fatty Acid Oxidation Disorders". Ultragenyx Pharmaceutical. 30 June 2020. Archived from the original on 1 July 2020. Retrieved 30 June 2020 – via GlobeNewswire.
- ↑ "Triheptanoin Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). 26 May 2006. Archived from the original on 3 July 2020. Retrieved 30 June 2020.
- ↑ "Triheptanoin Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). 1 February 2008. Archived from the original on 2 July 2020. Retrieved 30 June 2020.
- ↑ "Triheptanoin Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). 21 October 2014. Archived from the original on 2 July 2020. Retrieved 30 June 2020.
- ↑ "Triheptanoin Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). 15 April 2015. Archived from the original on 2 July 2020. Retrieved 30 June 2020.
- ↑ "EU/3/12/1081". European Medicines Agency (EMA). Archived from the original on 1 July 2020. Retrieved 30 June 2020.
- ↑ "EU/3/12/1082". European Medicines Agency (EMA). Archived from the original on 1 July 2020. Retrieved 30 June 2020.
- ↑ "EU/3/15/1495". European Medicines Agency (EMA). Archived from the original on 3 July 2020. Retrieved 30 June 2020.
- ↑ "EU/3/15/1508". European Medicines Agency (EMA). Archived from the original on 3 July 2020. Retrieved 30 June 2020.
- ↑ "EU/3/15/1524". European Medicines Agency (EMA). Archived from the original on 1 July 2020. Retrieved 30 June 2020.
- ↑ "EU/3/15/1525". European Medicines Agency (EMA). Archived from the original on 3 July 2020. Retrieved 30 June 2020.
- ↑ "EU/3/15/1526". European Medicines Agency (EMA). Archived from the original on 2 July 2020. Retrieved 30 June 2020.
- ↑ "EU/3/16/1710". European Medicines Agency (EMA). Archived from the original on 1 July 2020. Retrieved 30 June 2020.
- ↑ "Dojolvi: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 1 July 2020. Retrieved 30 June 2020.
- 1 2 3 4 5 6 7 8 9 10 11 12 "Drug Trials Snapshots: Dojolvi". U.S. Food and Drug Administration. 30 June 2020. Archived from the original on 8 August 2020. Retrieved 16 July 2020.
- ↑ World Health Organization (2019). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 82". WHO Drug Information. 33 (3): 694. hdl:10665/330879. License: CC BY-NC-SA 3.0 IGO.
Further reading
- de Almeida Rabello Oliveira M, da Rocha Ataíde T, de Oliveira SL, de Melo Lucena AL, de Lira CE, Soares AA, et al. (March 2008). "Effects of short-term and long-term treatment with medium- and long-chain triglycerides ketogenic diet on cortical spreading depression in young rats". Neurosci. Lett. 434 (1): 66–70. doi:10.1016/j.neulet.2008.01.032. PMID 18281154. S2CID 7754768.
- Mochel F, DeLonlay P, Touati G, Brunengraber H, Kinman RP, Rabier D, et al. (April 2005). "Pyruvate carboxylase deficiency: clinical and biochemical response to anaplerotic diet therapy". Mol. Genet. Metab. 84 (4): 305–12. doi:10.1016/j.ymgme.2004.09.007. PMID 15781190.
- Borges K, Sonnewald U (July 2012). "Triheptanoin--a medium chain triglyceride with odd chain fatty acids: a new anaplerotic anticonvulsant treatment?". Epilepsy Res. 100 (3): 239–44. doi:10.1016/j.eplepsyres.2011.05.023. PMC 3422680. PMID 21855298.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- Clinical trial number NCT01379625 for "Study of Triheptanoin for Treatment of Long-Chain Fatty Acid Oxidation Disorder (Triheptanoin)" at ClinicalTrials.gov