U0126
 | |
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
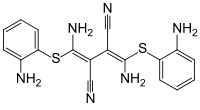
| Formula | C18H16N6S2 |
| Molar mass | 380.49 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
U0126 is a highly selective inhibitor of both MEK1 and MEK2, a type of MAPK/ERK kinase.[1][2] U0126 was found to functionally antagonize AP-1 transcriptional activity via noncompetitive inhibition of the dual specificity kinase MEK with IC50 of 72 nM for MEK1 and 58 nM for MEK2. U0126 inhibited anchorage-independent growth of Ki-ras-transformed rat fibroblasts by simultaneously blocking both extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin (mTOR)-p70(S6K) pathways.[3] The effects of U0126 on the growth of eight human breast cancer cell lines shown that U0126 selectively repressed anchorage-independent growth of MDA-MB231 and HBC4 cells, two lines with constitutively activated ERK.[4] Loss of contact with substratum triggers apoptosis in many normal cell types, a phenomenon termed anoikis. U0126 sensitized MDA-MB231 and HBC4 to anoikis, i.e., upon treatment with U0126, cells deprived of anchorage entered apoptosis.
U0126 is also a weak inhibitor of PKC, Raf, ERK, JNK, MEKK, MKK-3, MKK-4/SEK, MKK-6, Cdk2 and Cdk4.
Its potential for wiping long-term memories in rats has been studied at the Center for Neural Science at New York University.[5]
See also
- Xantocillin
References
- ↑ Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, et al. (July 1998). "Identification of a novel inhibitor of mitogen-activated protein kinase kinase". The Journal of Biological Chemistry. 273 (29): 18623–32. doi:10.1074/jbc.273.29.18623. PMID 9660836. S2CID 9896161.
- ↑ DeSilva DR, Jones EA, Favata MF, Jaffee BD, Magolda RL, Trzaskos JM, Scherle PA (May 1998). "Inhibition of mitogen-activated protein kinase kinase blocks T cell proliferation but does not induce or prevent anergy". Journal of Immunology. 160 (9): 4175–81. PMID 9574517.
- ↑ Duncia JV, Santella JB, Higley CA, Pitts WJ, Wityak J, Frietze WE, et al. (October 1998). "MEK inhibitors: the chemistry and biological activity of U0126, its analogs, and cyclization products". Bioorganic & Medicinal Chemistry Letters. 8 (20): 2839–44. doi:10.1016/s0960-894x(98)00522-8. PMID 9873633.
- ↑ Fukazawa H, Noguchi K, Murakami Y, Uehara Y (March 2002). "Mitogen-activated protein/extracellular signal-regulated kinase kinase (MEK) inhibitors restore anoikis sensitivity in human breast cancer cell lines with a constitutively activated extracellular-regulated kinase (ERK) pathway". Molecular Cancer Therapeutics. 1 (5): 303–9. PMID 12489846.
- ↑ Smith K (2007-03-11). "Wipe out a single memory". Nature Magazine. Retrieved 2017-12-31.