Vicenistatin
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C30H48N2O4 |
| Molar mass | 500.724 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
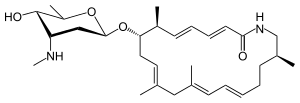
Vicenistatin is a macrolactam antibiotic synthesized by Streptomyces halstedii HC34. It was originally isolated from this bacterium in 1993.[1] It includes the unusual starter unit methylaspartate.[2]
References
- ↑ Shindo K, Kamishohara M, Odagawa A, Matsuoka M, Kawai H (July 1993). "Vicenistatin, a novel 20-membered macrocyclic lactam antitumor antibiotic". The Journal of Antibiotics. 46 (7): 1076–81. doi:10.7164/antibiotics.46.1076. PMID 8360102.
- ↑ Ogasawara Y, Kakinuma K, Eguchi T (July 2005). "Involvement of glutamate mutase in the biosynthesis of the unique starter unit of the macrolactam polyketide antibiotic vicenistatin". The Journal of Antibiotics. 58 (7): 468–72. doi:10.1038/ja.2005.62. PMID 16161486.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.