Virstatin
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H13NO4 |
| Molar mass | 283.283 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.40 g/cm3 |
| Melting point | 86–88 °C (187–190 °F) |
| Boiling point | 536.52 °C (997.74 °F) |
SMILES
| |
InChI
| |
Virstatin is a small molecule that inhibits the activity of the cholera protein, ToxT.[1]
Its activity in cholera was first published in 2005 in a paper that described the screening of a chemical library in a phenotypic screen and subsequent testing of one of the hits in infected mice.[1][2]
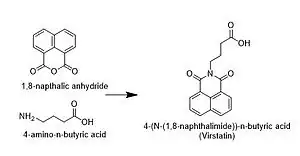
Reaction scheme used in original discovery and analysis of the molecule
The compound is an isoquinoline alkaloid[3] and can be synthesized by reacting 1,8-naphthalic anhydride with 4-amino-n-butyric acid[2] or by a simple two-step synthesis[4]
References
- 1 2 Anthouard R, DiRita VJ (February 2015). "Chemical biology applied to the study of bacterial pathogens". Infection and Immunity. 83 (2): 456–69. doi:10.1128/IAI.02021-14. PMC 4294262. PMID 25404026.
- 1 2 Hung DT, Shakhnovich EA, Pierson E, Mekalanos JJ (October 2005). "Small-molecule inhibitor of Vibrio cholerae virulence and intestinal colonization". Science. 310 (5748): 670–4. Bibcode:2005Sci...310..670H. doi:10.1126/science.1116739. PMID 16223984. S2CID 30557147.
- ↑ Cushnie TP, Cushnie B, Lamb AJ (November 2014). "Alkaloids: an overview of their antibacterial, antibiotic-enhancing and antivirulence activities". International Journal of Antimicrobial Agents. 44 (5): 377–86. doi:10.1016/j.ijantimicag.2014.06.001. PMID 25130096.
- ↑ McDonald CE (2009). "A Two-Step Synthesis of Virstatin, A Virulence Inhibitor of Vibrio cholerae". J. Chem. Educ. 86 (4): 482. Bibcode:2009JChEd..86..482M. doi:10.1021/ed086p482.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.