Voice prosthesis
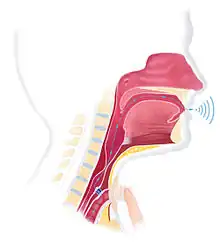
A voice prosthesis (plural prostheses) is an artificial device, usually made of silicone that is used in conjunction with voice therapy to help laryngectomized patients to speak. During a total laryngectomy, the entire voice box (larynx) is removed and the windpipe (trachea) and food pipe (esophagus) are separated from each other. During this operation an opening between the food pipe and the windpipe can be created (primary puncture). This opening can also be created at a later time (secondary puncture). This opening is called a tracheo-esophageal puncture (TE puncture). The voice prosthesis is placed in this opening. Then, it becomes possible to speak by occluding the stoma and blowing the air from the lungs through the inside of the voice prosthesis and through the throat, creating a voice sound, which is called tracheo-esophageal speech. The back end of the prosthesis sits at the food pipe. To avoid food, drinks, or saliva from coming through the prosthesis and into the lungs, the prosthesis has a small flap at the back. There are two ways of inserting the voice prosthesis: through the mouth and throat (retrograde manner) with the help of a guide wire, or directly through the tracheostoma (anterograde) manner. Nowadays, most voice prosthesis are placed anterograde, through the stoma.
History
There are two lines of development on behalf of giving a voice to patients being laryngectomized.
Artificial larynx
In 1869 the first artificial voice box (larynx) was built by Czermak.[1] In 1873 Theodor Billroth made a total laryngectomy (in steps) and implanted an artificial voice box.[1][2] A new development, using modern materials, took place in France by Debry.[3]
Voice prosthesis
In 1972 the very first voice prosthesis for voice rehabilitation after total laryngectomy was described in an article in Polish by Mozolewski.[4] Since then, many efforts have taken place in this area of rehabilitation. There are several manufacturers that have voice prosthesis in their product portfolio, e.g. Blom-Singer®, Adeva®, Eska®, MediTop®, Heimomed®. The internationally most widely used are devices made by InHealth Technologies and Atos Medical. In 1980, the first commercially available prosthesis was introduced by Singer and Blom:[5] the ‘Blom-Singer® Duckbill’, a 16 French diameter, non-flanged device that the patient could remove, clean and replace him- or herself (non-indwelling). The first indwelling voice prosthesis (Groningen Voice Prostheis) was described in 1984.[6] And in 1990, the first Provox® voice prosthesis, manufactured by Atos Medical, was introduced to the market,[7] followed by the Provox®2 in 1997,[8] the Provox® ActiValve™ in 2003,[9] and the non-indwelling Provox® NID™ in 2005.[10] 1994, the Blom-Singer® Classic™ Indwelling Voice Prostheses[11] was introduced to the market. Like the Blom-Singer® Advantage® Indwelling Voice Prosthesis it is only inserted or replaced by medical professionals, such as speech pathologists or physicians. In 2009 the third generation Provox® Vega[12] with SmartInserter™[13] was introduced.[14]
Non-indwelling and indwelling voice prostheses
The two main categories voice prosthesis can be divided into are ‘non-indwelling’ and ‘indwelling’ voice prostheses. ‘Non-indwelling’ voice prostheses can be replaced by the patients themselves,[10] whereas ‘indwelling’ prostheses have to be replaced by a medical professional. A non-indwelling voice prosthesis has a safety strap and may also have a string with a special safety medallion that is too wide to fall into the tracheostoma. It comes in different sizes and lengths and often has a noticeable color, e.g. blue or white to enhance visibility for self-replacement and maintenance. Disadvantages of the non-indwelling prosthesis are a certain amount of risk when inserting them by oneself and the devices have a shorter lifetime and need to be changed more often. In general it takes some practice to insert the prosthesis and the ability to take excellent care of the device. Indwelling devices have sturdier flanges and can only be replaced by a medical professional. The safety strap is cut off after insertion. The choice between ‘non-indwelling’ and ‘indwelling’ devices is really individual, depending on physical condition, maintenance and cost and can be alternated over a period of time to find out which one is most suitable.
Design of voice prostheses
The general design of voice prosthesis is quite consistent, even though there are unique characteristics. A voice prosthesis has retaining flanges at each end, the ‘tracheal flange’ and ‘esophageal flange’. Those flanges can vary in size and rigidity, e.g. indwelling prostheses have larger and more rigid flanges for stability and facilitate long-term placement. The flange near the food pipe (esophageal flange) is more rigid than the tracheal flange, near the windpipe. The one-way valve can be molded in one piece with the prosthesis and is often supported by a fluoroplastic valve seat (a colored ring that is tightly secured into the shaft of the prosthesis, adding rigidity and which is radiopaque). All voice prostheses have a safety strap, which is cut off in indwelling devices after the prosthesis is put in place; in non-indwelling devices the strap is not removed and is taped to the neck. A voice prosthesis has a one-way valve near the esophageal flange that enables pulmonary air to pass into the esophagus and pharynx for sound production and prevents content from the food pipe, such as liquids or saliva, from entering the trachea.
Properties of voice prostheses
Material
A voice prosthesis is usually made of medical grade silicone rubber. The valve flap and valve seat may be made out of silicone, fluoroplastic, or may be treated with SilverOxide.
Sizes
The size of a voice prosthesis varies in length, depending on the thickness of the wall between the foodpipe and the windpipe and thereby the length of the TE puncture. The according length of the voice prosthesis ranges usually between 4 and 22 mm. Another parameter is the outer diameter of the shaft of the voice prosthesis, ranging from 16-22.5 French. Which outer diameter is used is decided by the clinician and/or the patient and is most often a matter of personal preference. Studies have shown that a larger outer diameter of the voice prosthesis allows better airflow and thereby requires less effort to speak which has a positive effect on the overall voice quality.[15]
Problem-solving voice prosthesis for strong candida accumulation
There are even special, problem-solving voice prosthesis, e.g. for laryngectomized patients with very short device life times (less than 4 to 8 weeks) of their current prosthesis. Those extremely short device life times may be caused by excessive candida growth, so that the valve of the prosthesis does not close properly anymore and content from the food pipe leaks through the voice prosthesis into the windpipe. Some of these prostheses have a magnet within the valve that strengthens the closure, others are made of silver oxide silicone to reduce biofilm formation on the valve or use two valves, in case of defect of the esophageal valve, the other valve would seal. In patients with a short device life, the use of a voice prosthesis with supporting magnets in the valve seat and valve flap has been proven to be cost effective.[16]
Cleaning
It is important to clean the voice prosthesis regularly, as the silicone material is exposed to yeast (candida) and bacteria in the food pipe, which is normally present in these areas.[17] If yeast begins growing on or in the area of the valve flap of the voice prosthesis, it may not close well enough anymore. When this happens fluid starts to leak into the windpipe when eating or drinking.
Brushing
The inside of the voice prosthesis is usually cleaned with a brush, which removes food and mucus. It is recommended that the patient cleans the voice prosthesis regularly to keeps it open for speech and improve the device lifetime.
Flushing
It is also possible to flush the inside of the voice prosthesis with water or air.[18] Usually this is done in combination with brushing.
Leakage
A voice prosthesis has to be replaced regularly, because after a certain time, the valve flap of the voice prosthesis does not close properly anymore, due to yeast and other natural sediments. This causes leakage of saliva or drinks, which enters the wind pipe and makes the person concerned cough. If not resolved by cleaning the voice prosthesis, this is a sign that the voice prosthesis must be changed. If that is not possible right away, there are special plugs that can block the leakage. The patient can insert this plug themself before eating or drinking and remove it again afterwards, as speaking with the plug in place is usually not possible.
Device lifetime
The device lifetime can range from a couple of weeks up to two years, depending on individual circumstances.[19] The lifetime is influenced by daily food intake, especially dairy products,[20] but also radiotherapy[A 1] and GERD (Gastro Esophageal Reflux Disease) affects the voice prosthesis and its lifetime.[21] The cause of the prothesis' life time end is mostly leakage, but also growing of fistulae, granulation tissue, increasing valve's opening pressure and prothesis' loss [22]
Voice quality and speaking effort
The voice quality when speaking with a voice prosthesis is influenced by pulmonary support, airflow resistance of the voice prosthesis, and airflow resistance of the new voice source.[23] Although the voice prosthesis is only responsible for part of the total resistance – the neoglottis is responsible for the other part – favorable airflow characteristics are expected to enable the laryngectomized patient to speak with less effort.[12] The voice sounds rather clear though not very loud as samples show.[24]
Voice prosthesis and HME
An individual combination of voice prosthesis, heat and moisture exchanger after laryngectomy and attachment is important for good speech and pulmonary rehabilitation.[25] The HME sometimes is combined with free-hands-switch and virus and bacteria filter.[26]
Annotations
- ↑ A Free University of Berlin study shows a different result
References
- 1 2 Reutter, Sabine (2016). Prothetische Stimmrehabilitation nach totaler Kehlkopfentfernung - eine historische Abhandlung seit Billroth (1873) (Thesis) (in German). Universität Ulm. doi:10.18725/OPARU-1358. Retrieved 2017-01-08.
- ↑ "Tendances chirurgicales actuelles concernant la pose de l'implant phonatoire dans les laryngectomies totales, etmodalités de prise en charge orthophonique p. 8" (PDF) (in French). Retrieved 2017-01-18.
- ↑ Fréour, Pauline (2013-10-07). "Le premier larynx artificiel du monde est français". Le Figaro (in French). Retrieved 2017-01-14.
- ↑ Mozolewski, E. (1972). "Surgical rehabilitation of voice and speech following laryngectomy". Otolaryngologia Polska = the Polish Otolaryngology. 26(6): Otolaryngol Pol. 26 (6): 653–661. PMID 4654928.
{{cite journal}}: CS1 maint: location (link) - ↑ Singer, MI; Blom ED. (1980). "An endoscopic technique for restoration of voice after laryngectomy". Ann Otol Rhinol Laryngol. 89 (6 Pt 1): 529–533. doi:10.1177/000348948008900608. PMID 7458140. S2CID 10447001.
- ↑ Manni JJ, van den Broek P, de Groot MA, Berends E (1984). "Voice rehabilitation after laryngectomy with the Groningen prosthesis". J Otolaryngol. 13 (5): 333–336. PMID 6544851.
- ↑ Hilgers, FJ; Schouwenburg PF (1990). "A new low-resistance, self-retaining prosthesis (Provox) for voice rehabilitation after total laryngectomy". Laryngoscope. 100 (11): 1202–1207. doi:10.1288/00005537-199011000-00014. PMID 2233085.
- ↑ Hilgers, FJ; Ackerstaff AH; Balm AJ; Tan IB; Aaronson NK; Persson JO (1997). "Development and clinical evaluation of a second-generation voice prosthesis (Provox 2), designed for anterograde and retrograde insertion". Acta Otolaryngol. 117 (6): 889–896. doi:10.3109/00016489709114220. PMID 9442833.
- ↑ Hilgers FJ, Ackerstaff AH, Balm AJ, Van den Brekel MW, Bing Tan I, Persson JO (2003). "A new problem-solving indwelling voice prosthesis, eliminating the need for frequent Candida- and "underpressure"-related replacements: Provox ActiValve". Acta Otolaryngol. 123 (8): 972–979. doi:10.1080/00016480310015371. PMID 14606602. S2CID 24797860.
- 1 2 Hancock, K; Houghton B; As-Brooks CJ; Coman W (2005). "First clinical experience with a new non-indwelling voice prosthesis (Provox NID) for voice rehabilitation after total laryngectomy". Acta Otolaryngol. 125 (9): 981–990. doi:10.1080/00016480510043486. PMID 16109676. S2CID 10957879.
- ↑ Kress, P; Schafer P; Schwerdtfeger FP; Roesler S (2007). "Measurement and comparison of in vitro air-flow characteristics of the most frequently used European indwelling voice prostheses types". 6th European Congress of Oto-Rhino-Laryngology Head and Neck Surgery, June 30 - July 4, 2007, Vienna, Austria.
- 1 2 Hilgers FJ, Ackerstaff AH, et al. (2010). "Clinical phase I/feasibility study of the next generation indwelling Provox voice prosthesis (Provox Vega)". Acta Otolaryngol. 130 (4): 511–519. doi:10.3109/00016480903283766. PMID 19895334. S2CID 22637671.
- ↑ Hilgers, FJ; Ackerstaff AH; Jacobi I; Balm AJ; Tan IB; Van den Brekel MW (2010). "Prospective clinical phase II study of two new indwelling voice prostheses (Provox Vega 22.5 and 20 Fr) and a novel anterograde insertion device (Provox Smart Inserter)". Laryngoscope. 120 (6): 1135–1143. doi:10.1002/lary.20925. PMID 20513030. S2CID 206197136.
- ↑ Ward, EC; Hancock K; Lawson N; van As-Brooks CJ (2011). "Perceptual characteristics of tracheoesophageal speech production using the new indwelling Provox Vega voice prosthesis: a randomized controlled crossover trial". Head Neck. 33 (1): 13–19. doi:10.1002/hed.21389. PMID 20848411. S2CID 37769520.
- ↑ Hilgers FJ, Ackerstaff AH, et al. (2010). "Clinical phase I/feasibility study of the next generation indwelling Provox voice prosthesis (Provox Vega)". Acta Otolaryngol. 130 (4): 511–9. doi:10.3109/00016480903283766. PMID 19895334. S2CID 22637671.
- ↑ Soolsma, J; Van den Brekel MW; Ackerstaff AH; Balm AJ; Tan B; Hilgers FJ (2008). "Long-term results of Provox ActiValve, solving the problem of frequent candida- and "underpressure"-related voice prosthesis replacements" (PDF). Laryngoscope. 118 (2): 252–257. doi:10.1097/MLG.0b013e318159ebde. PMID 18090869. S2CID 221019846.
- ↑ Van Weissenbruch R, Albers FW, et al. (1997). "Deterioration of the Provox silicone tracheoesophageal voice prosthesis: microbial aspects and structural changes". Acta Otolaryngol. 117 (3): 452–458. doi:10.3109/00016489709113420. PMID 9199534.
- ↑ Free, RH; Van der Mei HC; Elving GJ; Van Weissenbruch R; Albers FW; Busscher HJ (2003). "Influence of the Provox Flush, blowing and imitated coughing on voice prosthetic biofilms in vitro". Acta Otolaryngol. 123 (4): 547–551. doi:10.1080/0036554021000028118. PMID 12797592. S2CID 35382053.
- ↑ Hilgers, FJ; Balm AJ (1993). "Long-term results of vocal rehabilitation after total laryngectomy with the low-resistance, indwelling Provox voice prosthesis system". Clin Otolaryngol. 18 (6): 517–523. doi:10.1111/j.1365-2273.1993.tb00627.x. PMID 8877233.
- ↑ Schwandt, LQ; van WR; Van der Mei HC; Busscher HJ; Albers FW (2005). "Effect of dairy products on the lifetime of Provox2 voice prostheses in vitro and in vivo". Head Neck. 27 (6): 471–477. CiteSeerX 10.1.1.631.6937. doi:10.1002/hed.20180. PMID 15825199. S2CID 9319432.
- ↑ Boscolo-Rizzo, P; Marchiori C; Gava A; Da Mosto MC (2008). "The impact of radiotherapy and GERD on in situ lifetime of indwelling voice prostheses". Eur Arch Otorhinolaryngol. 265 (7): 791–796. doi:10.1007/s00405-007-0536-1. PMID 18008081. S2CID 22109908.
- ↑ "r Lebensdauer von Stimmprothesen" (PDF) (in German). FU-Berlin. Retrieved 2017-03-10.
- ↑ Hilgers, FJ; Cornelissen MW; Balm AJ (1993). "Aerodynamic characteristics of the Provox low-resistance indwelling voice prosthesis". Eur Arch Otorhinolaryngol. 250 (7): 375–378. doi:10.1007/bf00180379. PMID 8286099. S2CID 38693652.
- ↑ "Speech smples". Retrieved 2017-03-11.
- ↑ Balle, VH; Rindso L; Thomsen JC (2000). "Primary speech restoration at laryngectomy by insertion of voice prosthesis-10 years' experience". Acta Otolaryngol Suppl. 120 (543): 244–245. doi:10.1080/000164800454512. PMID 10909032. S2CID 35289285.
- ↑ "Provox Micron HME". Retrieved 2017-03-11.