Limb development
| Development of the limbs | |
|---|---|
 Illustration of a human embryo at six weeks gestational age | |
 9-week human fetus from ectopic pregnancy | |
| Anatomical terminology |
Limb development in vertebrates is an area of active research in both developmental and evolutionary biology, with much of the latter work focused on the transition from fin to limb.[1]
Limb formation begins in the morphogenetic limb field, as mesenchymal cells from the lateral plate mesoderm proliferate to the point that they cause the ectoderm above to bulge out, forming a limb bud. Fibroblast growth factor (FGF) induces the formation of an organizer at the end of the limb bud, called the apical ectodermal ridge (AER), which guides further development and controls cell death. Programmed cell death is necessary to eliminate webbing between digits.
The limb field is a region specified by expression of certain Hox genes, a subset of homeotic genes, and T-box transcription factors – Tbx5 for forelimb or wing development, and Tbx4 for leg or hindlimb development. Establishment of the forelimb field (but not hindlimb field) requires retinoic acid signaling in the developing trunk of the embryo from which the limb buds emerge.[2][3] Also, although excess retinoic acid can alter limb patterning by ectopically activating Shh or Meis1/Meis2 expression, genetic studies in mouse that eliminate retinoic acid synthesis have shown that RA is not required for limb patterning.[4]
The limb bud remains active throughout much of limb development as it stimulates the creation and positive feedback retention of two signaling regions: the AER and its subsequent creation of the zone of polarizing activity (ZPA) with the mesenchymal cells.[5] In addition to the dorsal-ventral axis created by the ectodermal expression of competitive Wnt7a and BMP signals respectively, these AER and ZPA signaling centers are crucial to the proper formation of a limb that is correctly oriented with its corresponding axial polarity in the developing organism.[6][7] Because these signaling systems reciprocally sustain each other’s activity, limb development is essentially autonomous after these signaling regions have been established.[5]
Limb formation
Limb bud
Limb formation begins in the morphogenetic limb field. Limb formation results from a series of reciprocal tissue interactions between the mesenchyme of the lateral plate mesoderm and the overlying ectodermally derived epithelial cells. Cells from the lateral plate mesoderm and the myotome migrate to the limb field and proliferate to the point that they cause the ectoderm above to bulge out, forming the limb bud. The lateral plate cells produce the cartilaginous and skeletal portions of the limb while the myotome cells produce the muscle components.
The lateral plate mesodermal cells secrete fibroblast growth factors (FGF7 and FGF10) to induce the overlying ectoderm to form an organizer at the end of the limb bud, called the apical ectodermal ridge (AER), which guides further development and controls cell death.[8] The AER secretes further growth factors FGF8 and FGF4 which maintain the FGF10 signal and induce proliferation in the mesoderm. The position of FGF10 expression is regulated by two Wnt signaling pathways: Wnt8c in the hindlimb and Wnt2b in the forelimb. The forelimb and the hindlimb are specified by their position along the anterior/posterior axis and possibly by two transcription factors: Tbx5 and Tbx4, respectively.[9][10]
Precartilage condensations
The limb's skeletal elements are prefigured by tight aggregates known as cellular condensations of the pre-cartilage mesenchymal cells.[11] Mesenchymal condensation is mediated by extracellular matrix and cell adhesion molecules.[12] In the process of chondrogenesis, chondrocytes differentiate from the condensations to form cartilage, giving rise to the skeletal primordia. In the development of most vertebrate limbs (though not in some amphibians), the cartilage skeleton is replaced by bone later in development.
Periodicities of the limb pattern
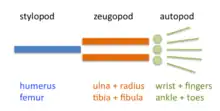
The limb is organized into three regions: stylopod, zeugopod, and autopod (in order from proximal to distal). The zeugopod and the autopod contain a number of periodic and quasi-periodic pattern motifs. The zeugopod consists of two parallel elements along the anteroposterior axis and the autopod contains 3-5 (in most cases) elements along the same axis. The digits also have a quasi-periodic arrangement along the proximodistal axis, consisting of tandem chains of skeletal elements. The generation of the basic limb plan during development results from the patterning of the mesenchyme by an interplay of factors that promote precartilage condensation and factors that inhibit it.[13]
The development of the basic limb plan is accompanied by the generation of local differences between the elements. For example, the radius and ulna of the forelimb, and the tibia and fibula of the hindlimb of the zeugopod are distinct from one another, as are the different fingers or toes in the autopod. These differences can be treated schematically by considering how they are reflected in each of the limb's three main axes.
A general consensus is that the patterning of the limb skeleton involves one or more Turing-type reaction–diffusion mechanisms.[1]
Evolution and development
The evolution of limbs from paired fins has been an area of much focus.[1] A reverse study of limb reduction and limb loss in the development of the snake is another active area of research.[14] It has been shown that there are conserved sequences involved in limb development retained in the genome of the snake. It is thought that these limb-enhancer sequences are conserved since there is overlap between those for limb development and those for phallus development (seen as a third type of appendage).[14][15] This aspect has also been studied in the mouse, where the usual limb-development signalling components are seen to play roles both in the development of the limbs and of the genital tubercle.[14][15] The study of limb reduction and limb loss is unravelling the genetic pathways that control limb development.[14]
The Turing system has enabled a number of possible outcomes in the evolutionary steps of patterning networks.[1]
Axial patterning and related issues
The developing limb has to align itself in relation to three axes of symmetry.[16] These are the craniocaudal (head to tail), dorsoventral (back to front), and proximodistal (near to far) axes.[16]
Many investigations into the development of the limb skeletal pattern have been influenced by the positional information concept proposed by Lewis Wolpert in 1971.[17] In tune with this idea, efforts have been made to identify diffusive signaling molecules (morphogens) that traverse orthogonal axes of developing limbs and determine locations and identities of skeletal elements in a concentration-dependent fashion.
Proximodistal patterning
Hox genes contribute to the specification of the stylopod, zeugopod and autopod. Mutations in Hox genes lead to proximodistal losses or abnormalities.[18] Three different models have been advanced for explaining the patterning of these regions.
Progress zone model
The apical ectodermal ridge (AER) creates and maintains a zone of cell proliferation known as the progress zone.[19] It is thought that cells here gain the positional information they need to travel to their destined position.[19] It was proposed that their positional value was determined by the length of time that the cells were in the progress zone but this has yet to be proved (as of 2001).[19] Proximal structures were proposed to be formed by the first cells to leave the zone and distal ones, by cells that left later.[19]
The Progress Zone model was proposed 30 years ago but recent evidence has conflicted with this model.[20]
Experimental evidence:
- Removing the AER at a later period of development results in less disruption of distal structures than if the AER was removed early in development.
- Grafting an early limb bud tip onto a late wing results in duplication of structures, while grafting a late wing bud tip onto an early limb results in a deletion of structures.
Early allocation and progenitor expansion model (or prespecification model)
Cells are specified for each segment in the early limb bud and this population of cells expand out as the limb bud grows. This model is consistent with the following observations. Cell division is seen throughout the limb bud. Cell death occurs within a 200 μm zone subjacent to the AER when it is removed; cell death forecloses some patterning. FGF-releasing beads are able to rescue limb development when the AER is removed by preventing this cell death.
Experimental evidence:
- Labeled cells in different position of an early limb bud were restricted to single segments of the limb.[21]
- Limbs lacking expression of required FGF4 & FGF8 showed all structures of the limb and not just the proximal parts.[22]
More recently, however, the investigators primarily responsible for both the Progress Zone and Prespecification models have acknowledged that neither of these models accounts adequately for the available experimental data.[20]
Turing-type reaction–diffusion model
This model, a reaction–diffusion model first proposed in 1979,[23] is based on the self-organizing properties of excitable media described by Alan Turing in 1952.[24] The excitable medium is the limb bud mesenchyme, in which cells interact by positively autoregulatory morphogens such as transforming growth factor beta (TGF-β) and inhibitory signaling pathways involving fibroblast growth factor (FGF) and Notch signalling. Proximodistal and craniocaudal axes are not considered to be independently specified, but instead emerge by transitions in the number of parallel elements as the undifferentiated apical zone of the growing limb bud undergoes reshaping.[25] This model only specifies a "bare bones" pattern. Other factors like sonic hedgehog (Shh) and Hox proteins, primary informational molecules in the other models, are proposed instead to play a fine-tuning role.
Experimental evidence:
- Limb mesenchymal cells, when dissociated and grown in culture or reintroduced within ectodermal "hulls" can recapitulate essential aspects of pattern formation, morphogenesis and differentiation.[26][27]
- Peculiarities of the limb skeletal pattern in the mouse Doublefoot mutant are predicted outcomes of a Turing-type mechanism.[28]
- Progressive reduction in distal Hox genes in a Gli3-null background results in progressively more severe polydactyly, displaying thinner and densely packed digits, suggesting (with the aid of computer modeling) that the dose of distal Hox genes modulates the period or wavelength of digits specified by a Turing-type mechanism.[29]
Craniocaudal patterning
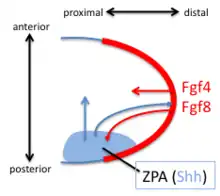
The discovery, in 1957, of the zone of polarizing activity (ZPA) in the limb bud provided a model for understanding the patterning activity by the action of a morphogenic gradient of sonic hedgehog (Shh).[30] Shh is recognised as a limb-specific enhancer.[15] Shh is both sufficient and necessary to create the ZPA and specify the craniocaudal pattern in the distal limb (Shh is not necessary for the polarity of the stylopod). Shh is turned on in the posterior through the early expression of Hoxd genes, the expression of Hoxb8, and the expression dHAND. Shh is maintained in the posterior through a feedback loop between the ZPA and the AER. Shh induces the AER to produce FGF4 and FGF8 which maintains the expression of Shh.
Digits 3,4 and 5 are specified by a temporal gradient of Shh. Digit 2 is specified by a long-range diffusible form of Shh and Digit 1 does not require Shh. Shh cleaves the Ci/Gli3 transcriptional repressor complex to convert the transcription factor Gli3 to an activator which activates the transcription of HoxD genes along the craniocaudal. Loss of the Gli3 repressor leads to the formation of generic (non-individualized) digits in extra quantities.[31]
Dorsoventral patterning
Dorsoventral patterning is mediated by Wnt7a signals in the overlying ectoderm not the mesoderm. Wnt7a is both necessary and sufficient to dorsalize the limb. Wnt7a also influences the craniocaudal and loss of Wnt7a causes the dorsal side of limbs to become ventral sides and causes missing posterior digits. Replacing Wnt7a signals rescues this defect. Wnt7a is also required to maintain expression of Shh.
Wnt-7a also causes Lmx-1, a Lim Homebox gene (and thus a transcription factor), to be expressed. Lmx-1 is involved in dorsalisation of the limb, which was shown by knocking out the Lmx-1 gene in mice.[32] The mice lacking the Lmx-1 produced ventral skin on both sides of their paws. There are other factors thought to control the DV patterning; Engrailed-1 represses the dorsalizing effect of Wnt-7a on the ventral side of the limbs.[33]
See also
- Holt–Oram syndrome
- Dysmelia
- LBH (gene)
References
- 1 2 3 4 Stewart, TA; Bhat, R; Newman, SA (2017). "The evolutionary origin of digit patterning". EvoDevo. 8: 21. doi:10.1186/s13227-017-0084-8. PMC 5697439. PMID 29201343.
- ↑ Stratford T, Horton C, Maden M (1996). "Retinoic acid is required for the initiation of outgrowth in the chick limb bud". Curr Biol. 6 (9): 1124–33. doi:10.1016/S0960-9822(02)70679-9. PMID 8805369. S2CID 14662908.
- ↑ Zhao X, Sirbu IO, Mic FA, et al. (June 2009). "Retinoic acid promotes limb induction through effects on body axis extension but is unnecessary for limb patterning". Curr. Biol. 19 (12): 1050–7. doi:10.1016/j.cub.2009.04.059. PMC 2701469. PMID 19464179.
- ↑ Cunningham, T.J.; Duester, G. (2015). "Mechanisms of retinoic acid signalling and its roles in organ and limb development". Nat. Rev. Mol. Cell Biol. 16 (2): 110–123. doi:10.1038/nrm3932. PMC 4636111. PMID 25560970.
- 1 2 Tickle, C (October 2015). "How the embryo makes a limb: determination, polarity and identity". Journal of Anatomy. 227 (4): 418–30. doi:10.1111/joa.12361. PMC 4580101. PMID 26249743.
- ↑ Parr, BA; McMahon, AP (23 March 1995). "Dorsalizing signal Wnt-7a required for normal polarity of D-V and A-P axes of mouse limb". Nature. 374 (6520): 350–3. Bibcode:1995Natur.374..350P. doi:10.1038/374350a0. PMID 7885472. S2CID 4254409.
- ↑ Pizette, S; Abate-Shen, C; Niswander, L (November 2001). "BMP controls proximodistal outgrowth, via induction of the apical ectodermal ridge, and dorsoventral patterning in the vertebrate limb". Development. 128 (22): 4463–74. doi:10.1242/dev.128.22.4463. PMID 11714672.
- ↑ Yonei-Tamura S, Endo T, Yajima H, Ohuchi H, Ide H, Tamura K (1999). "FGF7 and FGF10 directly induce the apical ectodermal ridge in chick embryos". Dev. Biol. 211 (1): 133–43. doi:10.1006/dbio.1999.9290. PMID 10373311.
- ↑ Ohuchi H, Takeuchi J, Yoshioka H, Ishimaru Y, Ogura K, Takahashi N, Ogura T, Noji S (1998). "Correlation of wing-leg identity in ectopic FGF-induced chimeric limbs with the differential expression of chick Tbx5 and Tbx4". Development. 125 (1): 51–60. doi:10.1242/dev.125.1.51. PMID 9389663.
- ↑ Rodriguez-Esteban C, Tsukui T, Yonei S, Magallon J, Tamura K, Izpisua Belmonte JC (1999). "The T-box genes Tbx4 and Tbx5 regulate limb outgrowth and identity". Nature. 398 (6730): 814–8. Bibcode:1999Natur.398..814R. doi:10.1038/19769. PMID 10235264. S2CID 4330287.
- ↑ DeLise, AM; Fischer, L; Tuan, RS (September 2000). "Cellular interactions and signaling in cartilage development". Osteoarthritis and Cartilage. 8 (5): 309–34. doi:10.1053/joca.1999.0306. PMID 10966838.
- ↑ Hall BK, Miyake T (2000). "All for one and one for all: condensations and the initiation of skeletal development". BioEssays. 22 (2): 138–47. doi:10.1002/(SICI)1521-1878(200002)22:2<138::AID-BIES5>3.0.CO;2-4. PMID 10655033.
- ↑ Newman SA, Bhat R (2007). "Activator-inhibitor dynamics of vertebrate limb pattern formation". Birth Defects Research Part C: Embryo Today: Reviews. 81 (4): 305–19. CiteSeerX 10.1.1.128.3260. doi:10.1002/bdrc.20112. PMID 18228262.
- 1 2 3 4 Infante, CR; Rasys, AM; Menke, DB (January 2018). "Appendages and gene regulatory networks: Lessons from the limbless". Genesis. 56 (1): e23078. doi:10.1002/dvg.23078. PMC 5783778. PMID 29076617.
- 1 2 3 Leal, F; Cohn, MJ (January 2018). "Developmental, genetic, and genomic insights into the evolutionary loss of limbs in snakes". Genesis. 56 (1): e23077. doi:10.1002/dvg.23077. PMID 29095557. S2CID 4510082.
- 1 2 Larsen, William (2001). Human embryology (3rd ed.). Philadelphia, Pa.: Churchill Livingstone. p. 335. ISBN 978-0-443-06583-5.
- ↑ Wolpert L (1971). Positional information and pattern formation. Curr Top Dev Biol. Current Topics in Developmental Biology. Vol. 6. pp. 183–224. doi:10.1016/S0070-2153(08)60641-9. ISBN 9780121531065. PMID 4950136.
- ↑ Wellik D, Capecchi M (2003). "Hox10 and Hox11 genes are required to globally pattern the mammalian skeleton". Science. 301 (5631): 363–7. Bibcode:2003Sci...301..363W. doi:10.1126/science.1085672. PMID 12869760. S2CID 9751891.
- 1 2 3 4 Larsen, William (2001). Human embryology (3rd ed.). Philadelphia, Pa.: Churchill Livingstone. p. 337. ISBN 978-0-443-06583-5.
- 1 2 Tabin C, Wolpert L (2007). "Rethinking the proximodistal axis of the vertebrate limb in the molecular era". Genes Dev. 21 (12): 1433–42. doi:10.1101/gad.1547407. PMID 17575045.
- ↑ Dudley A, Ros M, Tabin C (2002). "A re-examination of proximodistal patterning during vertebrate limb development". Nature. 418 (6897): 539–44. Bibcode:2002Natur.418..539D. doi:10.1038/nature00945. PMID 12152081. S2CID 1357061.
- ↑ Sun X, Mariani F, Martin G (2002). "Functions of FGF signalling from the apical ectodermal ridge in limb development". Nature. 418 (6897): 501–8. Bibcode:2002Natur.418..501S. doi:10.1038/nature00902. PMID 12152071. S2CID 4409248.
- ↑ Newman SA, Frisch HL (1979). "Dynamics of skeletal pattern formation in developing chick limb". Science. 205 (4407): 662–668. Bibcode:1979Sci...205..662N. doi:10.1126/science.462174. PMID 462174.
- ↑ Turing, AM (1952). "The Chemical Basis of Morphogenesis". Philosophical Transactions of the Royal Society B. 237 (641): 37–72. Bibcode:1952RSPTB.237...37T. doi:10.1098/rstb.1952.0012.
- ↑ Zhu J, Zhang YT, Alber MS, Newman SA (2010). "Bare bones pattern formation: a core regulatory network in varying geometries reproduces major features of vertebrate limb development and evolution". PLOS ONE. 5 (5): e:10892. Bibcode:2010PLoSO...510892Z. doi:10.1371/journal.pone.0010892. PMC 2878345. PMID 20531940.
- ↑ Moftah MZ, Downie SA, Bronstein NB, Mezentseva N, Pu J, Maher PA, Newman SA (2002). "Ectodermal FGFs induce perinodular inhibition of limb chondrogenesis in vitro and in vivo via FGF receptor 2". Dev. Biol. 249 (2): 270–82. doi:10.1006/dbio.2002.0766. PMID 12221006.
- ↑ Ros MA, Lyons GE, Mackem S, Fallon JF (1994). "Recombinant limbs as a model to study homeobox gene regulation during limb development". Dev. Biol. 166 (1): 59–72. doi:10.1006/dbio.1994.1296. PMID 7958460.
- ↑ Miura T, Shiota K, Morriss-Kay G, Maini PK (2006). "Mixed-mode pattern in Doublefoot mutant mouse limb−Turing reaction-diffusion model on a growing domain during limb development". J Theor Biol. 240 (4): 562–73. Bibcode:2006JThBi.240..562M. doi:10.1016/j.jtbi.2005.10.016. PMID 16364368.
- ↑ Sheth R, Marcon L, Bastida MF, Junco M, Quintana L, Dahn R, Kmita M, Sharpe J, Ros MA (2012). "Hox genes regulate digit patterning by controlling the wavelength of a Turing-type mechanism". Science. 338 (6113): 1476–1480. Bibcode:2012Sci...338.1476S. doi:10.1126/science.1226804. PMC 4486416. PMID 23239739.
- ↑ Zhu, J; Mackem, S (15 September 2017). "John Saunders' ZPA, Sonic hedgehog and digit identity - How does it really all work?". Developmental Biology. 429 (2): 391–400. doi:10.1016/j.ydbio.2017.02.001. PMC 5540801. PMID 28161524.
- ↑ Chiang C, Litingtung Y, Harris M, Simandl B, Li Y, Beachy P, Fallon J (2001). "Manifestation of the limb prepattern: limb development in the absence of sonic hedgehog function". Dev Biol. 236 (2): 421–35. doi:10.1006/dbio.2001.0346. PMID 11476582.
- ↑ Riddle RD, Ensini M, Nelson C, Tsuchida T, Jessell TM, Tabin C (1995). "'Induction of the LIM homeobox gene Lmx1 by WNT7a establishes dorsoventral pattern in the vertebrate limb". Cell. 83 (2): 631–40. doi:10.1016/0092-8674(95)90103-5. PMID 7585966. S2CID 16272219.
- ↑ "Archived copy" (PDF). Archived from the original (PDF) on 2008-08-28. Retrieved 2008-06-02.
{{cite web}}: CS1 maint: archived copy as title (link)