5N-Bicalutamide
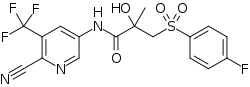 | |
| Clinical data | |
|---|---|
| Other names | 5-Azabicalutamide |
| Drug class | Nonsteroidal antiandrogen |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C17H13F4N3O4S |
| Molar mass | 431.36 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
5N-Bicalutamide, or 5-azabicalutamide, is a highly potent nonsteroidal antiandrogen (NSAA) which was discovered in 2016.[1][2] It is a structural modification of bicalutamide differing it from it only by the replacement of a carbon atom with a nitrogen atom in one of its phenyl rings.[1] Similarly to bicalutamide, the drug acts as a selective antagonist of the androgen receptor (AR).[1] However, unlike bicalutamide, it is a reversible covalent antagonist and stays bound to the receptor for a far longer amount of time.[1] As a result of this difference, 5N-bicalutamide has markedly improved potency relative to bicalutamide, with approximately 150-fold higher affinity for the AR (Ki = 0.15 nM versus 22.3 nM) and about 20-fold greater functional inhibition (IC50 = 15 nM versus 310 nM) of the AR.[1] Future studies of 5N-bicalutamide in normal and mutated prostate cancer cells are planned or underway and it is anticipated that N-bicalutamide may be able to overcome resistance to current antiandrogens that are used in the treatment of prostate cancer.[1]
Enzalutamide and related second-generation NSAAs like RD-162 and apalutamide were derived from bicalutamide and as a result are similar to it in chemical structure.[1] They have up to about 10-fold higher affinity for the AR than does bicalutamide and hence are comparatively more potent and efficacious antiandrogens.[1] However, their structures are rigidified such that the analogous structural modification that was done with bicalutamide to create 5N-bicalutamide could not be used to increase affinity or potency with them.[1] Enzalutamide was described in 2013 as "the emperor of all antiandrogens" and other second-generation NSAAs have similar potency to it,[3] so 5N-bicalutamide would appear to be among the most potent AR antagonists to have been developed thus far.[1]
See also
References
- 1 2 3 4 5 6 7 8 9 10 de Jesus Cortez F, Nguyen P, Truillet C, Tian B, Kuchenbecker KM, Evans MJ, Webb P, Jacobson MP, Fletterick RJ, England PM (2017). "Development of 5N-Bicalutamide, a High-Affinity Reversible Covalent Antiandrogen". ACS Chem. Biol. 12 (12): 2934–2939. doi:10.1021/acschembio.7b00702. PMID 28981251.
- ↑ Pamela, M., Fletterick, R. J., Kuchenbecker, K., & de Jesus Cortez, F. (2016). U.S. Patent Application No. 15/382,942. https://www.google.com/patents/US20170101384
- ↑ Antonarakis ES (2013). "Enzalutamide: The emperor of all anti-androgens". Transl Androl Urol. 2 (2): 119–120. doi:10.3978/j.issn.2223-4683.2012.09.04. PMC 3785324. PMID 24076589.