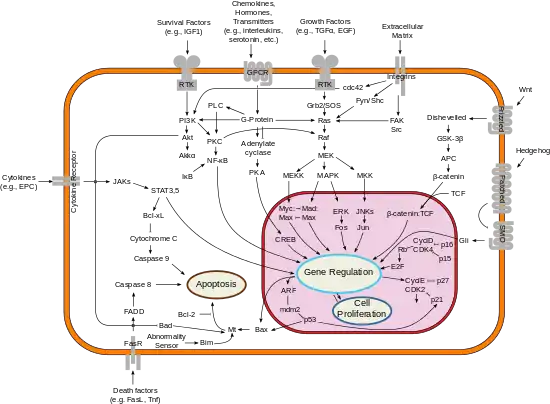
Bcl-xL

B-cell lymphoma-extra large (Bcl-xL), encoded by the BCL2-like 1 gene, is a transmembrane molecule in the mitochondria. It is a member of the Bcl-2 family of proteins, and acts as an anti-apoptotic protein by preventing the release of mitochondrial contents such as cytochrome c, which leads to caspase activation and ultimately, programmed cell death.[1]
Function
It is a well-established concept in the field of apoptosis that relative amounts of pro- and anti-survival Bcl-2 family of proteins determine whether the cell will undergo cell death; if more Bcl-xL is present, then pores are non-permeable to pro-apoptotic molecules and the cell survives. However, if Bax and Bak become activated, and Bcl-xL is sequestered away by gatekeeper BH3-only factors (e.g. Bim) causing a pore to form, cytochrome c is released leading to initiation of caspase cascade and apoptotic events.[2]
While the exact signaling pathway of Bcl-xL is still not known, it is believed that Bcl-xL differs highly from Bcl-2 in their mechanism of inducing apoptosis. Bcl-xL is about ten times more functional than Bcl-2 when induced by the chemotherapy drug, Doxorubicin[3] and can specifically bind to cytochrome C residues, preventing apoptosis.[4] It can also prevent the formation of Apaf-1 and Caspase 9 complex by acting directly upon Apaf-1 rather than Caspase 9, as shown in nematode homologs.[5]
Clinical significance
Bcl-xL dysfunction in mice can cause ineffective production of red blood cells, severe anemia, hemolysis, and death. This protein has also been shown as a requirement for heme production[6] and in erythroid lineage, Bcl-xL is a major survival factor responsible for an estimated half of the total survival "signal" proerythroblasts must receive in order to survive and become red cells. Bcl-xL promoter contains GATA-1 and Stat5 sites. This protein accumulates throughout the differentiation, ensuring the survival of erythroid progenitors. Because iron metabolism and incorporation into hemoglobin occurs inside the mitochondria, Bcl-xL was suggested to play additional roles in regulating this process in erythrocytes which could lead to a role in polycythemia vera, a disease where there is an overproduction of erythrocytes.[7]
Similar to other Bcl-2 family members, Bcl-xL has been implicated in the survival of cancer cells by inhibiting the function of p53, a tumor suppressor. In cancerous mouse cells, those which contained Bcl-xL were able to survive while those that only expressed p53 died in a small period of time.[8]
Bcl-xL is a target of various senolytic agents. Studies of cell cultures of senescent human umbilical vein endothelial cells have shown that both fisetin and quercetin induce apoptosis by inhibition of Bcl-xL.[9] Fisetin has roughly twice the senolytic potency as quercetin.[10]
Small molecule Bcl-xL inhibitor that directly binds to Bcl-xL and releases their partner such as Bax, a proapoptotic protein, has been suggested as an anticancer strategy via inducing of apoptosis.
Related proteins
Other Bcl-2 proteins include Bcl-2, Bcl-w, Bcl-xs, and Mcl-1.
References
- ↑ Korsmeyer SJ (March 1995). "Regulators of cell death". Trends in Genetics. 11 (3): 101–105. doi:10.1016/S0168-9525(00)89010-1. PMID 7732571.
- ↑ Finucane DM, Bossy-Wetzel E, Waterhouse NJ, Cotter TG, Green DR (January 1999). "Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xL". The Journal of Biological Chemistry. 274 (4): 2225–2233. doi:10.1074/jbc.274.4.2225. PMID 9890985.
- ↑ Fiebig AA, Zhu W, Hollerbach C, Leber B, Andrews DW (August 2006). "Bcl-XL is qualitatively different from and ten times more effective than Bcl-2 when expressed in a breast cancer cell line". BMC Cancer. 6 (213): 213. doi:10.1186/1471-2407-6-213. PMC 1560389. PMID 16928273.
- ↑ Bertini I, Chevance S, Del Conte R, Lalli D, Turano P (April 2011). "The anti-apoptotic Bcl-x(L) protein, a new piece in the puzzle of cytochrome c interactome". PLOS ONE. 6 (4): e18329. Bibcode:2011PLoSO...618329B. doi:10.1371/journal.pone.0018329. PMC 3080137. PMID 21533126.
- ↑ Hu Y, Benedict MA, Wu D, Inohara N, Núñez G (April 1998). "Bcl-XL interacts with Apaf-1 and inhibits Apaf-1-dependent caspase-9 activation". Proceedings of the National Academy of Sciences of the United States of America. 95 (8): 4386–4391. Bibcode:1998PNAS...95.4386H. doi:10.1073/pnas.95.8.4386. PMC 22498. PMID 9539746.
- ↑ Rhodes MM, Kopsombut P, Bondurant MC, Price JO, Koury MJ (September 2005). "Bcl-x(L) prevents apoptosis of late-stage erythroblasts but does not mediate the antiapoptotic effect of erythropoietin". Blood. 106 (5): 1857–1863. doi:10.1182/blood-2004-11-4344. PMC 1895223. PMID 15899920.
- ↑ Silva M, Richard C, Benito A, Sanz C, Olalla I, Fernández-Luna JL (February 1998). "Expression of Bcl-x in erythroid precursors from patients with polycythemia vera". The New England Journal of Medicine. 338 (9): 564–571. doi:10.1056/NEJM199802263380902. PMID 9475763.
- ↑ Schott AF, Apel IJ, Nuñez G, Clarke MF (October 1995). "Bcl-XL protects cancer cells from p53-mediated apoptosis". Oncogene. 11 (7): 1389–1394. PMID 7478561.
- ↑ Kirkland JL, Tchkonia T (November 2020). "Senolytic drugs: from discovery to translation". Journal of Internal Medicine. 288 (5): 518–536. doi:10.1111/joim.13141. PMC 7405395. PMID 32686219.
- ↑ Wyld L, Bellantuono I, Tchkonia T, Morgan J, Turner O, Foss F, et al. (July 2020). "Senescence and Cancer: A Review of Clinical Implications of Senescence and Senotherapies". Cancers. 12 (8): e2134. doi:10.3390/cancers12082134. PMC 7464619. PMID 32752135.