Beggiatoa
| Beggiatoa | |
|---|---|
 | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | Beggiatoa Trevisan 1842[1] |
| Species | |
|
Beggiatoa alba | |
Beggiatoa is a genus of Gammaproteobacteria belonging the order Thiotrichales, in the Proteobacteria phylum. This genus was one of the first bacteria discovered by Russian botanist Sergei Winogradsky. During his research in Anton de Bary’s laboratory of botany in 1887, he found that Beggiatoa oxidized hydrogen sulfide (H2S) as energy source, forming intracellular sulfur droplets, oxygen is the terminal electron acceptor and CO2 is used as carbon source. Winogradsky named it in honor of the Italian doctor and botanist Francesco Secondo Beggiato (1806 - 1883), from Venice.[3] Winogradsky referred to this form of metabolism as "inorgoxidation" (oxidation of inorganic compounds), today called chemolithotrophy. These organisms live in sulfur-rich environments such as soil, both marine and freshwater, in the deep sea hydrothermal vents and in polluted marine environments. The finding represented the first discovery of lithotrophy.[4][5] Two species of Beggiatoa have been formally described: the type species Beggiatoa alba and Beggiatoa leptomitoformis, the latter of which was only published in 2017.[2][6] This colorless and filamentous bacterium, sometimes in association with other sulfur bacteria (for example the genus Thiothrix), can be arranged in biofilm visible at naked eye formed by very long white filamentous mate, the white color is due to the stored sulfur. Species of Beggiatoa have cells up to 200 µ in diameter and they are one of the largest prokaryotes on Earth.[7]
Taxonomy
The genera Beggiatoa is a quiet diverse group as it has representatives occupying several habitats and niches, both in fresh and salt water. In the past they have been confused as close relatives of Oscillatoria spp. (Cyanobacteria) for the morphology and motility characters,[8] but 5S rRNA analysis showed that members of Beggiatoa are phylogenetically distant from Cyanobacteria being members of the Gammaproteobacteria phylum.[9]
The capability to oxidize sulfide and store sulfur are the main features which separates Beggiatoa and closely related Thioploca as filamentous colorless sulfur bacteria from other filamentous bacteria (like cyanobacteria and the nonsulfur-oxidizing Cytophaga and Flexibacter)[10] Another defining feature is the ability to store nitrate inside the vacuoles of the wide marine species’ cells. 16S rRNA sequences base studies inferred that this characteristic is shared between members of a monophyletic clade nested in the Beggiatoa genera; this clade also includes members of Thioploca and Thiomargarita, both presenting only slight differences with Beggiatoas: whereas the former grows sharing a common slime sheath, the latter has not conserved filamentous growth and forms chains of rounded cells. Since the phylogenic history do not reflect the nomenclature, there’s a need for a new denomination of genera and species.[11] The Neo-type strain is the B18LB and it settled the criteria for identification of the freshwater species Beggiatoa alba.[12]
According to NCBI database only two species of Beggiatoa spp. have been validly published: Beggiatoa alba, and Beggiatoa leptomitoformis.
Genetic
Because of the lack of pure culture, little is known about the genetic of Beggiatoa. Beggiatoa alba show a GC content between 40 and 42.7 mol%, the presence of two or three similar plasmids and the genome size of Beggiatoa alba strain B18LD result to be about 3Megabase (Mb).[7] An important study about the genomic of Beggiatoa analyzed the sequences obtained from two single filaments of a vacuolated strain. Optical mapping showed that the genome size was about 7.4Mb and the sequence analyses detected pathways for sulfur oxidation, nitrate and oxygen respiration, and CO2 fixation, confirming the chemolithoautotrophic physiology of Beggiatoa. Furthermore, comparative genomics indicates horizontal gene transfer between Beggiatoa and Cyanobacteria of storage, metabolic, and gliding abilities.[13]
Morphology and motility

By the observation of their morphological characteristics, Beggiatoa spp. can be divided into three categories:
- Freshwater strains, characterized by narrow filaments with no vacuoles;
- Narrow marine strains, without vacuoles (filaments' diameter of about 4.4 µm);
- Larger marine strains, with vacuoles for nitrate storing (filaments' diameter vary between 5 to 140 µm)
Obviously, this classification is purely ideal so some exceptions can exist. Narrow filaments are usually composed by cylindrical cells which length is about 1.5 to 8 times their thickness; wider filaments instead are disk-shaped with cell lengths from 0.10 to 0.90 times their cell width. In all of the cultured strains the terminal cells of the filaments appear rounded. Although they are Gram-negative bacteria, Beggiatoa show unusual cell-wall and membrane organization. Sometimes are present further membranes that cover the peptidoglycan layer and the number of this addictional membranes is very variable. Their presence maybe is due to the harsh conditions in which some of these organisms live. Even the intracellular granules can be covered by extra-membranes structure. Beside the sulfur granules, the cells often show the presence of similarly stored granules of polyhydroxybutyrate and polyphosphate. Very common in large marine vacuolated Beggiatoa are hollow-structured filaments, composed by cells with a narrow cytoplasm surrounding a large central vacuole, exploited for nitrate storing.[7][14]
The filaments move by gliding and this movement is likely connected to string-like structures in the outer membrane and trans-peptidoglycan channels. Sometimes the filaments can also break through the formation of necridia cell in the middle of the filament. The motility of the filament is very important for the adaptablity of the bacteria, because it allows to move on more suitable conditions for the cellular metabolism. The main drivers that guide the movement of Beggiatoa filaments are high oxygen and sulfide levels and light exposure, from which the filaments move away.[14]
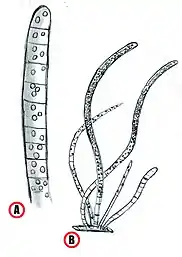
Cell growth
Beggiatoa reproducing strategy is fragmentation. The growth of a colony leading to mat development is obtained through alternating filament elongation and breakage. Breakage can happen essentially in the middle of a stretched filament, at the tip of a filament loop or where a tip of a loop was once placed. The presence of sacrificial cells is fundamental as they interrupt the communication between two parts of one filament; in this way each section can change its gliding direction causing the split.
The average filament length achieved through this process is also result of gene-environment interactions as, for instance, the growth and position of the filament is function of vertical gradients of oxygen and sulfide. Therefore, it is proposed that good environmental conditions will paradoxically cause cell death in order to enhance filament breakage, thus reproduction.[15]
Metabolism
Beggiatoa group is mainly composed by chemolithotrophic, sulfide-oxidizing bacteria. However, the range of possible metabolic pathways is very diversified, varying from the heterotrophy to the chemolithoautotrophy. Because of this huge variability the diverse bacteria of this genus can differ greatly from each other.[14]
Carbon metabolism
In Beggiatoa group are present both autotrophic and heterotrophic metabolisms. Autotrophic Beggiatoa carry out the CO2 fixation through the Calvin cycle and the employment of the RuBisCO enzyme. The latter shows different regulation levels in obligated and facultative autotrophs. For instance, in the obligately autotrophic strain MS-81-1c RuBisCO cannot be repressed, while in the facultatively autotrophic strain MS-81-6 it is tightly regulated to switch from autotrophic to heterotrophic growth and vice-versa. Beside the autotrophic strains, most of the freshwater Beggiatoa strains are heterotrophic, requiring organic substrates for growth. Specifically, many of them can be considered mixotrophs, because they grow heterotrophically, oxidizing organic compounds, but they can also use sulfide or other reduced sulfur compounds as electron donors. By this strategy, the organic carbon skeletons are saved for the purpose of increasing biomass and the CO2 autotrophic fixation is not required. Mixotrophy has been suspected to be the trophic modality for many freshwater strains, but it has only been found in one marine strain of Beggiatoa, MS-81-6.[14] Also a metabolic pathway of C-1 compounds utilization has been revealed in Beggiatoa leptomitoformis strain D-402, through comprehensive analysis of its genomic, bochemistry, physiology and molecular biology.[16]
Nitrogen metabolism
Beggiatoa group shows substantial versatility in utilizing nitrogen compounds. Nitrogen can be a source for growth or, in the case of nitrate, it can be an electron acceptor for anaerobic respiration. Heterotrophic freshwater Beggiatoa spp. assimilate nitrogen for growth. Nitrogen sources include nitrate, nitrite, ammonia, amino acids, urea, aspartate, asparagine, alanine and thiourea, depending on the capability of specific strains.
Autotrophic vacuolated Beggiatoa are able to store nitrate in their vacuoles 20.000 times the concentration of the surrounding sea water, and use it as terminal electron acceptor in anoxic conditions. This process, called Dissimilatory Nitrate Reduction to Ammonium (DNRA), reduces nitrate to ammonium. The capability of using nitrate as electron acceptor allows the colonization of anoxic environments, such as microbial mats and sediments. Several species are able to fix nitrogen using nitrogenase enzyme (e.g. Beggiatoa alba).[7][14]
Sulfur metabolism

One of the defining features of the genus Beggiatoa is the production of intracellular inclusions of sulfur resulting from the oxidation of reduced sulfur sources (e.g. hydrogen sulfide). In autotrophic Beggiatoa, sulfide is a source of energy and electrons for carbon fixation and growth. The oxidation of sulfide can be aerobic or anaerobic, in fact it can be coupled with the reduction of oxygen or with the reduction of nitrate. Sulfur produced by the oxidation of sulfide is stored into internal globules and can be used when the concentration of sulfide decreases. Thus, the temporarily storing of elemental sulfur (S0) increase the adaptability of an organism and its tolerance to changes in the concentrations of sulfide and oxygen.[7][14]
Sulfide aerobic oxidation:
Sulfide anaerobic oxidation:
There are some cases of chemoorganotrophy, too. For instance, the strain Beggiatoa sp. 35Flor usually do an aerobic respiration coupled with the oxidation of sulfide, but in anoxic condition a different type of respiration is activated. The energy is gained chemoorganotrophically from oxidation of PHA (polyhydroxyalkanoates), organic compounds previously synthesized through CO2 fixation during chemolithotrophic growth on oxygen and sulfide. In this case electron acceptor is the sulfur stored into the cell, so the final product is hydrogen sulfide.[17]
Anaerobic respiration:
Hydrogen metabolism
The strain Beggiatoa sp. 35Flor is able to use hydrogen as alternative electron donor to sulfide. This oxidation process can provide energy for maintenance and assimilatory purposes and is helpful to reduce stored sulfur when it becomes excessive, but it can’t provide growth to the strain.[18]
Hydrogen oxidation:
Phosphorus metabolism
Beggiatoa’s metabolism include the use of phosphorus in the polyphosphate form. The regulation of this metabolism relies on the environmental conditions. Oxygenated surroundings cause an accumulation of polyphosphate, while anoxia (coupled with an increasing concentration of sulfide) produces a breakdown of polyphosphate and its subsequent release from the cells. The released phosphate can then be deposited as phosphorite minerals in the sediments or stay dissolved in the water.[14]
Ecology
Filaments have been observed to form dense mats on sediments in a very huge variety of environments. They appear as a whitish layer and since they are present and flourish in marine environments which have been subject to pollution, they can be considered as an indicator species.[19] Beggiatoa and other related filamentous bacteria can cause settling problems in sewage treatment plants, industrial waste lagoons in canning, paper pulping, brewing, milling, causing the phenomenon called "bulking". Beggiatoa are also able to detoxify hydrogen sulfide in soil and have a role in the immobilization of heavy metals.[20][21]
Beggiatoa live at the oxic/anoxic interface, where they benefits from the presence of both hydrogen sulfide and oxygen. The chemolithoautotrophic strains of Beggiatoa are also considered important primary producers in dark environments.[7]
Habitat
The incredible number of adaptations and metabolisms of this genus of bacteria are consequences of the extraordinary environmental variability they can live in. Beggiatoa is almost benthic, it can be found in marine (Beggiatoa sp. MS-81-6 and MS-81-1c) or freshwater (Beggiatoa alba) environments and they only need sulfide or thiosulfide as electron donor and an oxidizer. They can usually be found in habitats that have high levels of hydrogen sulfide, these environments include cold seeps, sulfur springs, sewage contaminated water, mud layers of lakes, and near deep hydrothermal vents. Beggiatoa can also be found in the rhizosphere of swamp plants,[22][23] in soil, marine sediments and in the mangrove lagoon too (where they contribute to the lipid pool of the sediments[24]).[25] The freshwater species have typical habitats in sulfur springs, ditches, puddles, wetlands, lake sediments and in rice fields, where it can grow associated with the rice plants’ roots. The Beggiatoa that live in marine water can be found in regions where their source of energy (sulfide or thiosulfide) is available. It can be extracted from both inorganic or organic source and usually it is coupled with microoxic condition, therefore very low concentration of oxygen.[7] This genus of Gammaprotobacteria is also common in localizated area of anaerobic decomposition as whale carcass on the deep ocean seafloor.[7]

Vacuolated Beggiatoa can be very common in coastal upwelling regions (for example Peru and Chile coasts), deep sea hydrothermal vents and cold vents; in these environments the floc mats (hair-like breast) can grow up and cover large areas and reach the height of 30 cm. In deep sea hydrothermal vents and cold-seeps Beggiatoa can grow in filaments that can be up to 200 micrometres in diameter, which makes these ones the largest prokaryotes currently known. Vacuolated Beggiatoa can be found also in hypoxic seafloor, where the filaments can live inside the sediments at the depth of few cm (from 2 to 4 cm); in same cases the Beggiatoa bacterial filaments can be the most abundant part of the microbial biomass in the sediments.[7]
Beggiatoa can also be found in salt marshes, saline, and geothermally active underwater caves. Some studies on these environments have been carried out in the underwater caves of dolomitized limestone in Capo Palinuro, Salerno, (Italy). Here there are hydrothermal sulphidic springs and microbial biofilm is associated with the flow of hydrothermal fluids, whose activity is intermittent and starts during low tide. Mats found in the caves were composed by filaments resembling in most part Beggiatoa, Thiothrix and Flexibacter, and this Beggiatoa-like filaments were morphologically close to those found attached to rocks and the byssus of the mussels from Lucky Strike Hydrothermal vents on the Mid-Atlantic Ridge.[7]
Interactions with other organisms
Frequently, microorganisms of the genus Beggiatoa can form complex microbial mats, where they live in association with many other filamentous bacteria, such as cyanobacteria. The latter usually occupy the surface layer of the mat and during the day they produce a great amount of oxygen, derived from the photosynthetic activity. Conversely, Beggiatoa grow along an oxic/anoxic (oxygen/sulfide) interface, beneath the phototrophs, where they produce white patches.[7] However, during dark acclimation, the mat became anoxic, so the Beggiatoa moved to the mat surface, to avoid the high levels of H2S and remain at the oxygen/sulfide interface, while cyanobacteria remained in a dense layer below.[26] Sometimes Beggiatoa mats are enriched by the presence of diatoms and green euglenoids too,[20] but also protists as ciliates and dinoflagellates have been found associated with the mats at the Guaymas Basin hydrothermal vent ecosystem and they likely consume a large amount of bacterial biomass.[27]
As the microbial mats can reach 3 cm in width, they can represent a source of food for many grazers. This trophic connection has been observed in mangrove systems, where Beggiatoa cover part of marine sediments. It has been observed that these bacteria give an important contribution to meiofauna’s diet, in particular rotifers, polychaetes, nematodes and some groups of platyhelminthes, aschelminths and gnathostomulids.[28] A remarkable relationship has been found between nematodes and Beggiatoa. In fact nematodes seem to favor development of Beggiatoa mats, through the increasing of oxygen penetration and nutrient diffusion into the mat.[29]
Furthermore, many carrion appear covered by mats of Beggiatoa-like filamentous bacteria overlying anaerobic sulfate-reducing bacteria. They attract many metazoans scavengers, but when they break the mat, it releases hydrogen sulphide that drive away the scavengers. Hence, Beggiatoa can also be considered a carrion defence from the scavengers.[30]
Role in biogeochemical cycles

Several species of white sulfur bacteria in the family Beggiatoaceae are able to accumulate and transport NO3−, taken from shallow coastal sediments which is fundamental in metabolism, as well as accumulate it in filaments. The reduction of NO3− to ammonium implies the oxidation of H2S (except for geothermal vents, the sulphide derives from the underlying anaerobic sediment in which dissimilatory sulphate reduction occurs[20]): this reduction leads to the formation of suboxic zones characterized by positive redox potential and only trace concentrations of free H2S. In marine environment, the presence of these species is important because they have a fundamental role in regulation of the amount of H2S and NO3− :
- On the one hand, the regulation of free H2S concentration in marine sediments is fundamental because sulfide-depleted surface sediments are essential for survival of benthic infauna, in fact sulfide is highly toxic to bottom fauna and other organisms living in the sediment;
- On the other hand, NO3− reduction is important for the control of eutrophication in nitrogen-limited coastal waters.[31]
Beggiatoa can also accumulate phosphorus as polyphosphate and it subsequently releases phosphate in anoxic conditions. This might increase the availability of phosphorus to primary producers if the phosphate is released from the sediment to the water column. Some studies about the phosphorus cycling and the release of phosphorus linked to Beggiatoa have been realized in Baltic Sea. These studies showed that the reduction of sulfide by these bacteria may decrease the rate of iron sulfide formation in the sediments, and thus increase the phosphorus retention capability of the sediment.[14]
Cultivation
Selective Enrichments
The most successful enrichments for Beggiatoa spp. have been made using a shallow pan or aquarium in which it has been added few centimeters of sand, different amount of CaSO4 and K2HPO4, a source of complex organic polymers such as seaweed, several centimeters of sulfide-rich marine mud and seawater. The enrichment must contain the proper sulfide-oxygen interface that can be possible only if air is introduced, for example, by a slow steady flow of freshly aerated seawater. Another type of enrichment associated with Beggiatoa spp. is based on the use of extracted dried grass or hay in a mineral medium because the complex polymers such as cellulose residues in the material provide sulfate reduction. This also provides the hydrogen sulfide necessary to enrich for Beggiatoa.[7]
Pure Culture Isolation
There are three different possible techniques to obtain isolated Beggiatoa strains in pure culture:
- Isolation on agar plates
- Isolation using liquid media
- Isolation and cultivation in gradient media
Isolation on agar plates

The procedure that allows to obtain isolated heterotrophic strain requires an agar plate containing dilute organic substrate such as small amount of peptone. Then, it’s necessary to collect tufts of Beggiatoa filaments from the environment, to wash them using sterile washing solution and to place them on the agar plate. In this way there will be some growing filaments moving away from the central inoculum that can be used as inoculum for a new agar plate. For the isolation of marine Beggiatoa strains (that show autotrophic growth), since they are obligate microaereophiles it’s essential to provide micro-oxic conditions and to use particular agar plates made by filtered seawater supplemented with sodium sulfide and sodium acetate. On the contrary, for the freshwater strains isolation it’s necessary to perform under oxic conditions (air atmosphere) using a variety of media containing a low concentration of single organic compound such as acetate, Na2S or thiosulfate.[7]
Isolation using liquid media
Liquid media are often used for enrichment, MPN enumeration and bulk cultivation of Beggiatoa. In order to have a successful cultivation of heterotrophic or mixotrophic freshwater Beggiatoa, liquid media has to contain little amounts of carbon substrate, either soil extracts or acetate. The type species and strain (Beggiatoa alba str. B18LD) and related strains are generally grown in media that include a salt base, acetate as carbon source, and variable yeast extract and sulfide additions.[32] Some marine autotrophic Beggiatoa strains are also been cultured on defined liquid mineral medium with thiosulfate, CO2, micro-oxic conditions under aeration with 0.25% O2 (v/v) in the gas phase.[7]
Isolation and cultivation in gradient media
Autotrophic strains coming from a single filament isolation on agar can easily be maintained and propagated in sulfide gradient tubes in which sulfide-rich agar plugs are overlaid with sulfide-free soft agar. Tubs are loosely closed in order to permit the exchange of headspace gasses with the atmosphere. As result, two opposite layers are formed, one that contains sulfide while the other one oxygen: this allows the growth of a well-defined Beggiatoa layer at the sulfide-oxygen interface. The gradient medium construction requires different amounts of J3 medium (made by agar and NaHCO3) supplemented with neutralized Na2S placed in a screw-capped tube. Here, the sulfur source is provided by the flux of sulfide. Another ‘’ layer ‘’ is made by NaHCO3 without sulfide or thiosulfate: all of the sulfide will be below the interface between the sulfidic agar plug and the sulfide-free overlay agar while there will be another layer in the top of the tube that represents the oxygen reservoir. It begins to form a gradient shape due to the reaction between sulfide and oxygen: as results, the filaments rapidly proliferate at the sulfide-oxygen interface, forming a marked layer or “plate,” of 1 mm but it’s also possible to appreciate that these bacteria can track the interface and slowly descend owing to the gradual depletion of the sulfide reservoir.[7]
References
- ↑ Trevisan V (1842). "Prospetto della Flora Euganea". Coi Tipi Del Seminario. Padova. pp. 1–68.
- 1 2 Parte AC. "Beggiatoa". LPSN.
- ↑ Burkhardt, Lotte (2022). Eine Enzyklopädie zu eponymischen Pflanzennamen [Encyclopedia of eponymic plant names] (pdf) (in German). Berlin: Botanic Garden and Botanical Museum, Freie Universität Berlin. doi:10.3372/epolist2022. ISBN 978-3-946292-41-8. Retrieved January 27, 2022.
- ↑ Ljungdahl LG (2003). Biochemistry and physiology of anaerobic bacteria. Springer. p. 17. ISBN 978-0-387-95592-6.
- ↑ Mukhopadhyaya PN, Deb C, Lahiri C, Roy P (August 2000). "A soxA gene, encoding a diheme cytochrome c, and a sox locus, essential for sulfur oxidation in a new sulfur lithotrophic bacterium". Journal of Bacteriology. 182 (15): 4278–87. doi:10.1128/JB.182.15.4278-4287.2000. PMC 101942. PMID 10894738.
- ↑ Dubinina G, Savvichev A, Orlova M, Gavrish E, Verbarg S, Grabovich M (February 2017). "Beggiatoa leptomitoformis sp. nov., the first freshwater member of the genus capable of chemolithoautotrophic growth". International Journal of Systematic and Evolutionary Microbiology. 67 (2): 197–204. doi:10.1099/ijsem.0.001584. PMID 27902215.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Teske A, Nelson DC (2006). "The Genera Beggiatoa and Thioploca". In Dworkin M, Falkow S, Rosenberg E, Schleifer KH (eds.). The Prokaryotes. Springer New York. pp. 784–810. doi:10.1007/0-387-30746-x_27. ISBN 978-0-387-25496-8.
- ↑ Reichenbach H (1981-10-01). "Taxonomy of the gliding bacteria". Annual Review of Microbiology. 35 (1): 339–64. doi:10.1146/annurev.mi.35.100181.002011. PMID 6794424.
- ↑ Stahl DA, Lane DJ, Olsen GJ, Heller DJ, Schmidt TM, Pace NR (1987). "Phylogenetic Analysis of Certain Sulfide-Oxidizing and Related Morphologically Conspicuous Bacteria by 5S Ribosomal Ribonucleic Acid Sequences". International Journal of Systematic and Evolutionary Microbiology. 37 (2): 116–122. doi:10.1099/00207713-37-2-116. ISSN 1466-5026.
- ↑ Teske A, Nelson DC (2006). "The Genera Beggiatoa and Thioploca". In Dworkin M, Falkow S, Rosenberg E, Schleifer KH (eds.). The Prokaryotes. The Prokaryotes: Volume 6: Proteobacteria: Gamma Subclass. New York, NY: Springer. pp. 784–810. doi:10.1007/0-387-30746-x_27. ISBN 978-0-387-30746-6. PDF.
- ↑ Ahmad A, Kalanetra KM, Nelson DC (June 2006). "Cultivated Beggiatoa spp. define the phylogenetic root of morphologically diverse, noncultured, vacuolate sulfur bacteria". Canadian Journal of Microbiology. 52 (6): 591–8. doi:10.1139/w05-154. PMID 16788728.
- ↑ Boone DR, Brenner DJ, Castenholz RW, De Vos P, Garrity GM, Krieg NR, Goodfellow N (2001–2012). Bergey's manual of systematic bacteriology (2nd ed.). New York: Springer. ISBN 978-0-387-21609-6. OCLC 619443681.
- ↑ Mussmann M, Hu FZ, Richter M, de Beer D, Preisler A, Jørgensen BB, et al. (September 2007). Moran NA (ed.). "Insights into the genome of large sulfur bacteria revealed by analysis of single filaments". PLOS Biology. 5 (9): e230. doi:10.1371/journal.pbio.0050230. PMC 1951784. PMID 17760503.
- 1 2 3 4 5 6 7 8 Ruuskanen M (2014). "The genus Beggiatoa and its effects on the nutrient cycles of the Baltic Sea". doi:10.13140/RG.2.1.4814.6329.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Kamp A, Røy H, Schulz-Vogt HN (October 2008). "Video-supported analysis of Beggiatoa filament growth, breakage, and movement". Microbial Ecology. 56 (3): 484–91. doi:10.1007/s00248-008-9367-x. PMC 2755761. PMID 18335158.
- ↑ Orlova MV, Tarlachkov SV, Kulinchenko EI, Dubinina GA, Tutukina MN, Grabovich MY (December 2018). "Beggiatoa leptomitoformis D-402". Indian Journal of Microbiology. 58 (4): 415–422. doi:10.1007/s12088-018-0737-x. PMC 6141403. PMID 30262951.
- ↑ Schwedt A, Kreutzmann AC, Polerecky L, Schulz-Vogt HN (2012). "Sulfur respiration in a marine chemolithoautotrophic beggiatoa strain". Frontiers in Microbiology. 2: 276. doi:10.3389/fmicb.2011.00276. PMC 3253548. PMID 22291687.
- ↑ Kreutzmann AC, Schulz-Vogt HN (April 2016). Löffler FE (ed.). "Oxidation of Molecular Hydrogen by a Chemolithoautotrophic Beggiatoa Strain". Applied and Environmental Microbiology. 82 (8): 2527–36. Bibcode:2016ApEnM..82.2527K. doi:10.1128/AEM.03818-15. PMC 4959497. PMID 26896131.
- ↑ Elliott JK, Spear E, Wyllie-Echeverria S (2006). "Mats of Beggiatoa bacteria reveal that organic pollution from lumber mills inhibits growth of Zostera marina". Marine Ecology. 27 (4): 372–380. Bibcode:2006MarEc..27..372E. doi:10.1111/j.1439-0485.2006.00100.x. ISSN 1439-0485.
- 1 2 3 Fenchel T, Bernard C (1995). "Mats of colourless sulphur bacteria. I. Major microbial processes". Marine Ecology Progress Series. 128: 161–170. Bibcode:1995MEPS..128..161F. doi:10.3354/meps128161. ISSN 0171-8630.
- ↑ Drewniak L, Krawczyk PS, Mielnicki S, Adamska D, Sobczak A, Lipinski L, et al. (2016-08-10). "Physiological and Metagenomic Analyses of Microbial Mats Involved in Self-Purification of Mine Waters Contaminated with Heavy Metals". Frontiers in Microbiology. 7: 1252. doi:10.3389/fmicb.2016.01252. PMC 4978725. PMID 27559332.
- ↑ Dudley M. "Beggiatoa". Virginia Tech. Archived from the original on 2009-02-07.
- ↑ Ahmad A, Barry JP, Nelson DC (January 1999). "Phylogenetic affinity of a wide, vacuolate, nitrate-accumulating Beggiatoa sp. from Monterey Canyon, California, with Thioploca spp". Applied and Environmental Microbiology. 65 (1): 270–7. Bibcode:1999ApEnM..65..270A. doi:10.1128/AEM.65.1.270-277.1999. PMC 91012. PMID 9872789.
- ↑ SamKamaleson A, Gonsalves MJ (April 2019). "Role of sulfur-oxidizing bacteria on the ecology in tropical mangrove sediments". Regional Studies in Marine Science. 28: 100574. doi:10.1016/j.rsma.2019.100574. ISSN 2352-4855. S2CID 134524779.
- ↑ Jean MR, Gonzalez-Rizzo S, Gauffre-Autelin P, Lengger SK, Schouten S, Gros O (2015-02-17). Kellogg CA (ed.). "Two new Beggiatoa species inhabiting marine mangrove sediments in the Caribbean". PLOS ONE. 10 (2): e0117832. Bibcode:2015PLoSO..1017832J. doi:10.1371/journal.pone.0117832. PMC 4331518. PMID 25689402.
- ↑ Lichtenberg M, Cartaxana P, Kühl M (2020). "Vertical Migration Optimizes Photosynthetic Efficiency of Motile Cyanobacteria in a Coastal Microbial Mat". Frontiers in Marine Science. 7. doi:10.3389/fmars.2020.00359. ISSN 2296-7745. S2CID 218863468.
- ↑ Pasulka A, Hu SK, Countway PD, Coyne KJ, Cary SC, Heidelberg KB, Caron DA (July 2019). "SSU-rRNA Gene Sequencing Survey of Benthic Microbial Eukaryotes from Guaymas Basin Hydrothermal Vent". The Journal of Eukaryotic Microbiology. 66 (4): 637–653. doi:10.1111/jeu.12711. PMID 30620427. S2CID 58616192.
- ↑ Pascal PY, Dubois S, Boschker HT, Gros O (2014-12-03). "Trophic role of large benthic sulfur bacteria in mangrove sediment" (PDF). Marine Ecology Progress Series. 516: 127–138. Bibcode:2014MEPS..516..127P. doi:10.3354/meps11035. ISSN 0171-8630.
- ↑ Salvadó H, Palomo A, Mas M, Puigagut J, Gracia M (May 2004). "Dynamics of nematodes in a high organic loading rotating biological contactors". Water Research. 38 (10): 2571–8. doi:10.1016/j.watres.2004.03.007. PMID 15159160.
- ↑ Dayton PK, Oliver JS, Thrush SF, Hammerstrom K (February 2019). "Bacteria defend carrion from scavengers". Antarctic Science. 31 (1): 13–15. Bibcode:2019AntSc..31...13D. doi:10.1017/S0954102018000457. ISSN 0954-1020. S2CID 134564783.
- ↑ Sayama M, Risgaard-Petersen N, Nielsen LP, Fossing H, Christensen PB (November 2005). "Impact of bacterial NO3(-) transport on sediment biogeochemistry". Applied and Environmental Microbiology. 71 (11): 7575–7. doi:10.1128/AEM.71.11.7575-7577.2005. PMC 1287653. PMID 16269807.
- ↑ Schmidt TM, Arieli B, Cohen Y, Padan E, Strohl WR (December 1987). "Sulfur metabolism in Beggiatoa alba". Journal of Bacteriology. 169 (12): 5466–72. doi:10.1128/jb.169.12.5466-5472.1987. PMC 213973. PMID 3316186.