CUMYL-PEGACLONE
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
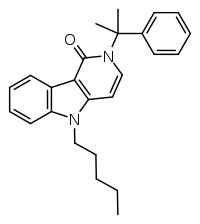
| Formula | C25H28N2O |
| Molar mass | 372.5 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
CUMYL-PEGACLONE (SGT-151) is a gamma-carboline based synthetic cannabinoid that has been sold as a designer drug.[1][2][3][4][5][6] The gamma-carboline core structure seen in CUMYL-PEGACLONE had not previously been encountered in a designer cannabinoid, though it is similar in structure to other gamma-carboline cannabinoids disclosed by Bristol-Myers Squibb in 2001.[7][8][9]
Legal status
Sweden's public health agency classified CUMYL-PEGACLONE as a narcotic substance, on January 18, 2019.[10]
See also
References
- ↑ Ernst L, Brandhorst K, Papke U, Altrogge A, Zodel S, Langer N, Beuerle T (August 2017). "Identification and quantification of synthetic cannabinoids in 'spice-like' herbal mixtures: Update of the German situation in early 2017". Forensic Science International. 277: 51–58. doi:10.1016/j.forsciint.2017.05.019. PMID 28601726.
- ↑ Angerer V, Mogler L, Steitz JP, Bisel P, Hess C, Schoeder CT, Müller CE, Huppertz LM, Westphal F, Schäper J, Auwärter V (July 2017). "Structural characterization and pharmacological evaluation of the new synthetic cannabinoid CUMYL-PEGACLONE". Drug Testing and Analysis. 10 (3): 597–603. doi:10.1002/dta.2237. PMID 28670781.
- ↑ Mogler L, Wilde M, Huppertz LM, Weinfurtner G, Franz F, Auwärter V (January 2018). "Phase I metabolism of the recently emerged synthetic cannabinoid CUMYL-PEGACLONE and detection in human urine samples". Drug Testing and Analysis. 10 (5): 886–891. doi:10.1002/dta.2352. PMID 29314750.
- ↑ Halter S, Angerer V, Röhrich J, Groth O, Roider G, Hermanns-Clausen M, Auwärter V (February 2019). "Cumyl-PEGACLONE: A comparatively safe new synthetic cannabinoid receptor agonist entering the NPS market?". Drug Testing and Analysis. 11 (2): 347–349. doi:10.1002/dta.2545. PMID 30468574.
- ↑ Janssens L, Cannaert A, Connolly MJ, Liu H, Stove CP (September 2020). "In vitro activity profiling of Cumyl-PEGACLONE variants at the CB1 receptor: Fluorination versus isomer exploration". Drug Testing and Analysis. 12 (9): 1336–1343. doi:10.1002/dta.2870. hdl:1854/LU-8687072. PMID 32490586. S2CID 219285656.
- ↑ Tiemensma M, Rutherford JD, Scott T, Karch S (November 2020). "Emergence of Cumyl-PEGACLONE-related fatalities in the Northern Territory of Australia". Forensic Science, Medicine, and Pathology. 17 (1): 3–9. doi:10.1007/s12024-020-00334-0. PMID 33185835. S2CID 226309264.
- ↑ WO application 2001058869, Leftheris K, Zhao, R, Chen BC, Kiener P, Wu H, Pandit C, Chennagiri R, Wrobleski S, Chen P, Hynes j, Longphre M, Norris D, Spergel S, Tokarski J, "Cannabinoid Receptor Modulators, Their Processes of Preparation, and Use of Cannabinoid Receptor Modulators in Treating Respiratory and Non-Respiratory Diseases", published 16 AUGUST 2001, assigned to Bristol-Myers Squibb Company
- ↑ Wrobleski ST, Chen P, Hynes J, Lin S, Norris DJ, Pandit CR, Spergel S, Wu H, Tokarski JS, Chen X, Gillooly KM, Kiener PA, McIntyre KW, Patil-Koota V, Shuster DJ, Turk LA, Yang G, Leftheris K (May 2003). "Rational design and synthesis of an orally active indolopyridone as a novel conformationally constrained cannabinoid ligand possessing antiinflammatory properties". Journal of Medicinal Chemistry. 46 (11): 2110–6. doi:10.1021/jm020329q. PMID 12747783.
- ↑ {{cite journal | vauthors = Alam RM, Keating JJ | title = Adding more "spice" to the pot: A review of the chemistry and pharmacology of newly emerging heterocyclic synthetic cannabinoid receptor agonists | journal = Drug Testing and Analysis | volume = 12 | issue = 3 | pages = 297–315 | date = March 2020 | pmid = 31854124 | doi = 10.1002/dta.2752 }
- ↑ "Sexton nya ämnen klassas som narkotika eller hälsofarlig vara" (in Swedish). Folkhälsomyndigheten. 18 January 2019.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.