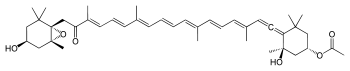
Fucoxanthin
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(1S,3R,4M)-3-Hydroxy-4-{(3E,5E,7E,9E,11E,13E,15E,17E)-18-[(1S,4S,6R)-4-hydroxy-2,2,6-trimethyl-7-oxabicyclo[4.1.0]heptan-1-yl]-3,7,12,16-tetramethyl-17-oxooctadeca-1,3,5,7,9,11,13,15,17-nonaen-1-ylidene}-3,5,5-trimethylcyclohexyl acetate | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| 3DMet | |
Beilstein Reference |
6580822 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.212.315 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C42H58O6 |
| Molar mass | 658.920 g·mol−1 |
| Hazards | |
| GHS labelling: | |
Pictograms |
 |
Signal word |
Warning |
Hazard statements |
H319 |
Precautionary statements |
P264, P280, P305+P351+P338, P337+P313 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Fucoxanthin is a xanthophyll, with formula C42H58O6. It is found as an accessory pigment in the chloroplasts of brown algae and most other heterokonts, giving them a brown or olive-green color. Fucoxanthin absorbs light primarily in the blue-green to yellow-green part of the visible spectrum, peaking at around 510-525 nm by various estimates and absorbing significantly in the range of 450 to 540 nm.
Function
Carotenoids are pigments produced by plants and algae and play a role in light harvesting as part of the photosynthesis process. Xanthophylls are a subset of carotenoids, identified by the fact that they are oxygenated either as hydroxyl groups or as epoxide bridges. This makes them more water soluble than carotenes like beta-carotene. Fucoxanthin is a xanthophyll that contributes more than 10% of the estimated total production of carotenoids in nature.[1] It is an accessory pigment found in the chloroplasts of many brown macroalgae, such as Fucus spp., and the golden-brown unicellular microalgae, the diatoms. It absorbs blue and green light at bandwidth 450-540 nm, imparting a brownish-olive color to algae. Fucoxanthin has a highly unique structure that contains both an epoxide bond and hydroxyl groups along with an allenic bond (carbon-carbon double bond) and a conjugated carbonyl group (carbon-oxygen double bond) in the polyene chain. All of these features provide fucoxanthin with powerful antioxidant activity.[2]
In macroalgal plastids, fucoxanthin acts like an antenna for light harvesting and energy transfer in the photosystem light harvesting complexes.[3] In diatoms like Phaeodactylum tricornutum, fucoxanthin is protein-bound along with chlorophyll to form a light harvesting protein complex.[4] Fucoxanthin is the dominant carotenoid, responsible for up to 60% of the energy transfer to chlorophyll a in diatoms [5] When bound to protein, the absorption spectrum of fucoxanthin expands from 450-540 nm to 390-580 nm, a range that is useful in aquatic environments.[6]
Sources
Fucoxanthin is present in brown seaweeds and diatoms and was first isolated from Fucus, Dictyota, and Laminaria by Willstätter and Page in 1914.[7] Seaweeds are commonly consumed in south-east Asia and certain countries in Europe, while diatoms are single-cell planktonic microalgae characterized by a golden-brown color, due to their high content of Fucoxanthin. Generally, diatoms contain up to 4 times more Fucoxanthin than seaweed, making diatoms a viable source for fucoxanthin industrially.[8] Diatoms can be grown in controlled environments (such as photobioreactors). Brown seaweeds are mostly grown in the open sea, often exposed to metals and metalloids.[9]
Potential therapeutic applications
Fucoxanthin has been shown to induce G1 cell-cycle arrest and apoptosis in various cancer cell lines and tumor growth in animal models of cancer.[10][11] Fucoxanthin also reduces weight, improves blood lipid profiles, and decreased insulin resistance in animal models of obesity.[12][13][14] In a human clinical trial Fucoxanthin was shown to improve weight parameters in slightly obese Japanese subjects.[15] In nonclinical assessments, fucoxanthin showed the capacity to notably inhibit the growth of Mycobacterium tuberculosis. Its mechanism of action was found to be correlated to the ability to inactivate two vital enzymes that play a significant role in mycobacterial cell wall biosynthesis namely UDP-galactopyranose mutase (UGM) and arylamine-N-acetyltransferase (TBNAT).[16]
Bioavailability and safety
Limited studies of the bioavailability of fucoxanthin in humans suggest that it is low but might be improved through formulation.[17] In rodents, fucoxanthin displays low toxicity when administered orally.[17] While human safety data is limited, the FDA has acknowledged the use of Fucoxanthin as a dietary supplement and filled a New Dietary Ingredient (NDI) notification of Fucoxanthin derived from the microalgae Phaeodactylum tricornutum.[18]
See also
- Chlorophyll
References
- ↑ Dembitsky VM, Maoka T (November 2007). "Allenic and cumulenic lipids". Progress in Lipid Research. 46 (6): 328–75. doi:10.1016/j.plipres.2007.07.001. PMID 17765976.
- ↑ Hu T, Liu D, Chen Y, Wu J, Wang S (March 2010). "Antioxidant activity of sulfated polysaccharide fractions extracted from Undaria pinnitafida in vitro". International Journal of Biological Macromolecules. 46 (2): 193–8. doi:10.1016/j.ijbiomac.2009.12.004. PMID 20025899.
- ↑ Owens TG, Wold ER (March 1986). "Light-Harvesting Function in the Diatom Phaeodactylum tricornutum: I. Isolation and Characterization of Pigment-Protein Complexes". Plant Physiology. 80 (3): 732–8. doi:10.1104/pp.80.3.732. PMC 1075192. PMID 16664694.
- ↑ Guglielmi G, Lavaud J, Rousseau B, Etienne AL, Houmard J, Ruban AV (September 2005). "The light-harvesting antenna of the diatom Phaeodactylum tricornutum. Evidence for a diadinoxanthin-binding subcomplex" (PDF). The FEBS Journal. 272 (17): 4339–48. doi:10.1111/j.1742-4658.2005.04846.x. PMID 16128804.
- ↑ Papagiannakis E, van Stokkum IH, Fey H, Büchel C, van Grondelle R (November 2005). "Spectroscopic characterization of the excitation energy transfer in the fucoxanthin-chlorophyll protein of diatoms". Photosynthesis Research. 86 (1–2): 241–50. doi:10.1007/s11120-005-1003-8. PMID 16172942.
- ↑ Premvardhan L, Sandberg DJ, Fey H, Birge RR, Büchel C, van Grondelle R (September 2008). "The charge-transfer properties of the S2 state of fucoxanthin in solution and in fucoxanthin chlorophyll-a/c2 protein (FCP) based on stark spectroscopy and molecular-orbital theory". The Journal of Physical Chemistry B. 112 (37): 11838–53. doi:10.1021/jp802689p. PMC 2844098. PMID 18722413.
- ↑ Peng J, Yuan JP, Wu CF, Wang JH (2011-10-10). "Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: metabolism and bioactivities relevant to human health". Marine Drugs. 9 (10): 1806–28. doi:10.3390/md9101806. PMC 3210606. PMID 22072997.
- ↑ Wang LJ, Fan Y, Parsons RL, Hu GR, Zhang PY, Li FL (January 2018). "A Rapid Method for the Determination of Fucoxanthin in Diatom". Marine Drugs. 16 (1): 33. doi:10.3390/md16010033. PMC 5793081. PMID 29361768.
- ↑ Li H, Ji H, Shi C, Gao Y, Zhang Y, Xu X, Ding H, Tang L, Xing Y (April 2017). "Distribution of heavy metals and metalloids in bulk and particle size fractions of soils from coal-mine brownfield and implications on human health". Chemosphere. 172: 505–515. doi:10.1016/j.chemosphere.2017.01.021. PMID 28104559.
- ↑ Satomi Y (April 2017). "Antitumor and Cancer-preventative Function of Fucoxanthin: A Marine Carotenoid". Anticancer Research. 37 (4): 1557–1562. doi:10.21873/anticanres.11484. PMID 28373414.
- ↑ Martin LJ (July 2015). "Fucoxanthin and Its Metabolite Fucoxanthinol in Cancer Prevention and Treatment". Marine Drugs. 13 (8): 4784–98. doi:10.3390/md13084784. PMC 4557004. PMID 26264004.
- ↑ Muradian K, Vaiserman A, Min KJ, Fraifeld VE (October 2015). "Fucoxanthin and lipid metabolism: A minireview". Nutrition, Metabolism, and Cardiovascular Diseases. 25 (10): 891–7. doi:10.1016/j.numecd.2015.05.010. PMID 26141943.
- ↑ Gammone MA, D'Orazio N (April 2015). "Anti-obesity activity of the marine carotenoid fucoxanthin". Marine Drugs. 13 (4): 2196–214. doi:10.3390/md13042196. PMC 4413207. PMID 25871295.
- ↑ Maeda H (2015). "Nutraceutical effects of fucoxanthin for obesity and diabetes therapy: a review". Journal of Oleo Science. 64 (2): 125–32. doi:10.5650/jos.ess14226. PMID 25748372.
- ↑ Hitoe, Shoketsu; Shimoda, Hiroshi (30 April 2017). "Seaweed Fucoxanthin Supplementation Improves Obesity Parameters in Mild Obese Japanese Subjects". Functional Foods in Health and Disease. 7 (4): 246. doi:10.31989/ffhd.v7i4.333.
- ↑ Šudomová, Miroslava; Shariati, Mohammad; Echeverría, Javier; Berindan-Neagoe, Ioana; Nabavi, Seyed; Hassan, Sherif (14 November 2019). "A Microbiological, Toxicological, and Biochemical Study of the Effects of Fucoxanthin, a Marine Carotenoid, on Mycobacterium tuberculosis and the Enzymes Implicated in Its Cell Wall: A Link Between Mycobacterial Infection and Autoimmune Diseases". Marine Drugs. 17 (11): 641. doi:10.3390/md17110641. PMC 6891772. PMID 31739453.
- 1 2 Peng J, Yuan JP, Wu CF, Wang JH (2011). "Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: metabolism and bioactivities relevant to human health". Marine Drugs. 9 (10): 1806–28. doi:10.3390/md9101806. PMC 3210606. PMID 22072997.
- ↑ "NDI 1048 - Fucoxanthin from Algatechnologies". www.regulations.gov. Food and Drug Administration. May 25, 2018.
Other studies
- Haugan, J (1994). "Isolation and characterisation of four allenic (6'S)-isomers of fucoxanthin". Tetrahedron Letters. 35 (14): 2245–2248. doi:10.1016/S0040-4039(00)76810-9.